Abstract
Background
Several studies conducted in a laboratory-related environment have shown that exercise is associated with increased muscle quality in older adults. The aim of the present study was to investigate whether recreational exercise may also be associated with muscle quality in men and women aged 50 years and over.
Methods
Data are from 312 individuals (215 women) aged 50 years and older. Body composition (dual-energy X-ray absorptiometry) and knee extension strength (KES) of the right leg (one repetition maximum) were assessed. Muscle quality (MQ) (KES/right lower limb lean mass) was calculated. Recreational exercises (duration and weekly amount) were determined by structured interview.
Results
The duration of the period during which participants practiced resistance activities was the only predictor of MQ (p = 0.018) and explained an additional 1.6 % of the variance in MQ, after controlling for age and gender. Furthermore, the weekly amount of practice of aerobic activities significantly interacted with age (p < 0.001) to determine MQ.
Conclusions
Findings suggest that long-term engagement in resistance exercise is beneficial for muscle quality and should be encouraged. Furthermore, beyond 60 years, aerobic activities also seem to be positively associated with muscle quality.
Keywords: Resistance training, Recreational exercise, Muscle mass, Muscle strength, Physical activity
Introduction
A number of studies investigated the relationship between muscle quality (MQ) (strength per unit of muscle) and physical function, some showing positive correlations [1-4], while others do not [5, 6]. In an attempt to clarify these findings, we showed that this relationship was complex and influenced by at least three factors: lean body mass, the degree of obesity (as assessed by body mass index (BMI)), and age [7]. These results highlighted that it was important to preserve MQ with increasing age and BMI in order to maintain functional capacity. Therefore, preventing the negative effects of age on MQ [8, 9] can only benefit older adults. It is not clear when the decline in muscle quality really becomes significant, but previous studies show that the loss of muscle mass and strength (i.e., the two components of muscle quality) is already evident at the fifth decade and then becomes even more pronounced [10, 11].
The strategy most commonly used to improve MQ in elderly is exercise, especially resistance training [12-15]. Nine to 14 weeks of resistance training have been reported to improve MQ by 14 to 28 % in both men and women [12-15]. In addition, Kennis et al. [16] observed that 1-year fitness or vibration trainings enhanced MQ (+11 and +7 %, respectively). Chastin et al. [17] also reported that alternating periods of intense activity and long recovery times were associated with MQ in men.
With the exception of the work of Chastin et al. [17], all interventions mentioned above [12-16] were conducted in the context of laboratory-related exercise programs. These programs were designed to be reproducible and effective, that is, of a sufficient level of difficulty to maximize the probability of observing significant effects, while limiting the choice of practice, attendance, repetition, intensity, etc. However, outside of this ideal context, exercises can be quite different. It is recognized that elderly individuals are very sensitive to factors that may hamper their involvement in exercise. Eighty-seven percent of them have at least one barrier to prohibit exercise participation [18]. Fears of incurring injury or pain, exacerbation of illness or disease, questions over the expected benefits of exercise, or being “too old” are frequently cited reasons for not engaging in exercise [19]. Even among those engaged in exercise, only 30 % of older men and 15 % of older women actually sustain regular activities [20]. Therefore, it is legitimate to question the actual benefits of exercise in these conditions. Consequently, the aim of the present analysis was to investigate the relationship between recreational exercise and muscle quality in men and women aged 50 years and older.
Methods
Study population and procedure
Five hundred and twenty-five registered members of the YMCAs of Montreal (164 men and 361 women) aged 50 years and over (50–89 years) volunteered to participate in this study. The minimum age was set at 50 since the decline in muscle function becomes apparent at this age [10, 11]. To be included in the study, participants had to (a) be registered at the YMCA, (b) live and get around autonomously in the community, and (c) be able to understand and answer the questionnaires. Participants were excluded if used walking aid. All procedures were approved by the ethics committee of the University of Quebec at Montreal. All participants were fully informed about the nature, goal, procedures, and risks of the study, and they gave their informed consent.
After screening for the aforementioned inclusion criteria, participants were invited to visit their YMCA center where their muscle strength and physical function were assessed. They were then invited for a visit at the University of Quebec at Montreal where their body composition was assessed using dual-energy X-ray absorptiometry (DXA). Two hundred participants chose not to take the DXA and were excluded from these analyses. Thirteen additional participants were excluded because of missing data on muscle strength assessment. The present analyses were limited to the 312 (97 men and 215 women) remaining participants.
Anthropometric measurement and body composition assessment
Body weight (BW) was measured using an electronic scale (Tanita BC-558, Tanita, Arlington Heights, IL). Height was measured using a stadiometer (Seca, Hanover, MD) attached to the wall. Body mass index [BMI = BW (kg)/height (m2)] was calculated. Lean body mass (LBM) was evaluated by DXA (version 6.10.019; General Electric Lunar Corporation, Madison, WI). Appendicular lean body mass (AppLBM) [sum of upper and lower limb lean body masses (in kg)] and appendicular lean body mass index [AppLBMI = AppLBM (kg)/height2 (m2)] were calculated.
Muscle quality calculation
Lower limb MQ was calculated in three steps, as described elsewhere [7]: in step 1, the maximum KES of the participants’ right leg was determined by one repetition maximum (RM) on a standard Atlantis C-105 knee extension machine (in kg) and multiplied by 9.81 to be expressed in Newton (N); in step 2, the right lower limb LBM was evaluated by DXA; and in step 3, muscle quality was finally calculated by dividing KES of the right leg by right lower limb LBM (in N/kg LBM).
Exercise habits
Recreational exercise habits (planned, structured, and repetitive bodily movement done to improve or maintain one or more components of physical fitness) have been identified using structured interview conducted by a trained kinesiologist using a form comprising activities available at the YMCAs and blank spaces to allow recording of unlisted activities. This assessment method was chosen over more objective methods (e.g., accelerometry) because (1) it allows to cover and identify all exercises (e.g., resistance training, walking, swimming, martial arts, yoga, etc.), (2) it allows to collect accurate information for each activity, and (3) it is clinically very accessible.
Participants were asked to specify the exercise time (in min/week) for each activity in which they were currently engaged, either inside or outside the YMCAs, and for how long these activities have been practiced (in months). Activities were then categorized in three main categories: resistance, aerobic, and body and mind exercises. Based on this information, we calculated the weekly exercise time (WET) as well as the average exercise duration (AED) in total or by category of activity.
Potential confounders
Physical examination and health status questionnaires were used to record comorbid conditions (hypertension, diabetes, cancer, stroke, or other diseases). Cognitive impairment was assessed with the Montreal Cognitive Assessment (MoCA) questionnaire, and a score less than 26 was considered low [21]. Sociodemographic information (level of education, smoking habits, etc.) was also noted.
Statistical analysis
Hierarchical multiple regression analysis was used to analyze the association between exercise characteristics and MQ. Bivariate tests were first conducted to determine which variable to include in the regression analysis. Gender (t = −5.480, p < 0.001) and age (t = −0.329, p < 0.001), but not BMI, were associated with MQ and considered as a control variables. Among the exercise characteristics (total, resistance, aerobic, and body and mind WET or AED), only resistance AED was associated with MQ (r = 0.179, p = 0.003). No comorbidity (present or past cancer, cardiovascular disease, hypertension, arthritis, back pain, and cognition) was associated with MQ. In spite of gender differences in MQ, there was no evidence that the association between exercise characteristics and MQ differed by gender (p values from gender interaction tests were ˃0.05). We thus decided not to conduct sex-stratified analysis.
Preliminary analyses were conducted to ensure that the assumptions of normality, linearity, multicollinearity, and homoscedasticity were not violated. Hierarchical regressions were also used to investigate the relationship between resistance AED, right leg KES, and LBM. We then tested the hypothesis that age may interact with other physical activity characteristics (total or categorical WET or AED). To do so, following the method of Aiken and West [22], variables [age and exercise characteristics (total or categorical WET or AED)] were centered at the mean and entered in regression analyses (as well as their interaction term). Analyses were performed using SPSS 17.0 (Chicago, IL). A p value less than 0.05 was considered statistically significant.
Results
Participant’s characteristics
Participant’s characteristics are presented in Table 1. Briefly, body weight, BMI, AppLBMI, right lower limb LBM, KES, and MQ were higher in men than in women (p < 0.001). Body and mind AED was also higher in men than in women (p = 0.030). The reason why a disproportionate number of women volunteered to participate in the study is not obvious, but one may hypothesize that it may originate from gender differences in exercise motivation and expectation in terms of physical health, so that the topic of this study (physical activity, body composition, and physical function) is closer to women’s concerns. As for example, women appear to be more likely than men to report falls, seek medical care, and/or discuss falls and fall prevention with health-care provider [23].
Table 1.
Participants’ characteristics
| Variable | Men (n = 97) | Women (n = 215) | p |
|---|---|---|---|
| Age | 61 ± 8 | 61 ± 2 | 0.68 |
| Anthropometric measures | |||
| Body weight (kg) | 81.4 ± 15.2 | 66.8 ± 11.5 | <0.001 |
| BMI (kg/m2) | 27.0 ± 4.2 | 25.7 ± 4.4 | 0.024 |
| Muscle mass and function | |||
| AppLBMI (kg/m2) | 9.2 ± 0.9 | 7.3 ± 0.8 | <0.001 |
| Right leg LBM (kg) | 10.4 ± 1.5 | 7.3 ± 1.1 | <0.001 |
| KES (N) | 340 ± 130 | 177 ± 98 | <0.001 |
| Muscle quality (N/kg) | 32 ± 12 | 24 ± 13 | <0.001 |
| Exercise characteristicsa | |||
| Physically active (%) | 99.0 | 97.7 | 0.44 |
| Total WET (min/week) | 522 ± 332 | 499 ± 278 | 0.52 |
| Total AED (months) | 118 ± 102 | 90 ± 78 | 0.021 |
| Practicing resistance activities (%) | 68.0 | 63.3 | 0.41 |
| Resistance WET (min/week) | 128 ± 83 | 109 ± 71 | 0.090 |
| Resistance AED (months) | 81 ± 108 | 52 ± 83 | 0.060 |
| Practicing aerobic activities (%) | 99.0 | 95.3 | 0.11 |
| Aerobic WET (min/week) | 394 ± 283 | 378 ± 261 | 0.62 |
| Aerobic AED (months) | 137 ± 122 | 113 ± 113 | 0.09 |
| Practicing body and mind activities (%) | 32.0 | 43.3 | 0.059 |
| Body and mind WET (min/week) | 123 ± 120 | 135 ± 82 | 0.62 |
| Body and mind AED (months) | 90 ± 116 | 42 ± 52 | 0.030 |
| Smoking habits (%) | |||
| Current smoking | 7.4 | 6.0 | 0.82 |
| Past smoking | 60.9 | 62.6 | 0.91 |
| Education level (%) | |||
| Primary school | 3.1 | 5.1 | 0.72 |
| High school | 9.3 | 9.3 | |
| Postgraduate degree | 87.6 | 85.5 | |
| Comorbidities (%) | |||
| Present or past cancer | 2.1 | 14.9 | <0.001 |
| Cardiovascular disease | 17.7 | 12.9 | 0.29 |
| Hypertension | 26.3 | 20.5 | 0.30 |
| Arthritis | 41.7 | 48.4 | 0.33 |
| Back pain | 29.9 | 26.5 | 0.59 |
| Cognitive impairment | 17.9 | 11.0 | 0.10 |
BMI body mass index, AppLBMI appendicular lean body mass index, LBM lean body mass, KES knee extension strength, WET weekly exercise time, AED average exercise duration
aDescriptive analyses were conducted in individuals practicing the physical activity in question
Relationship between exercise and muscle quality
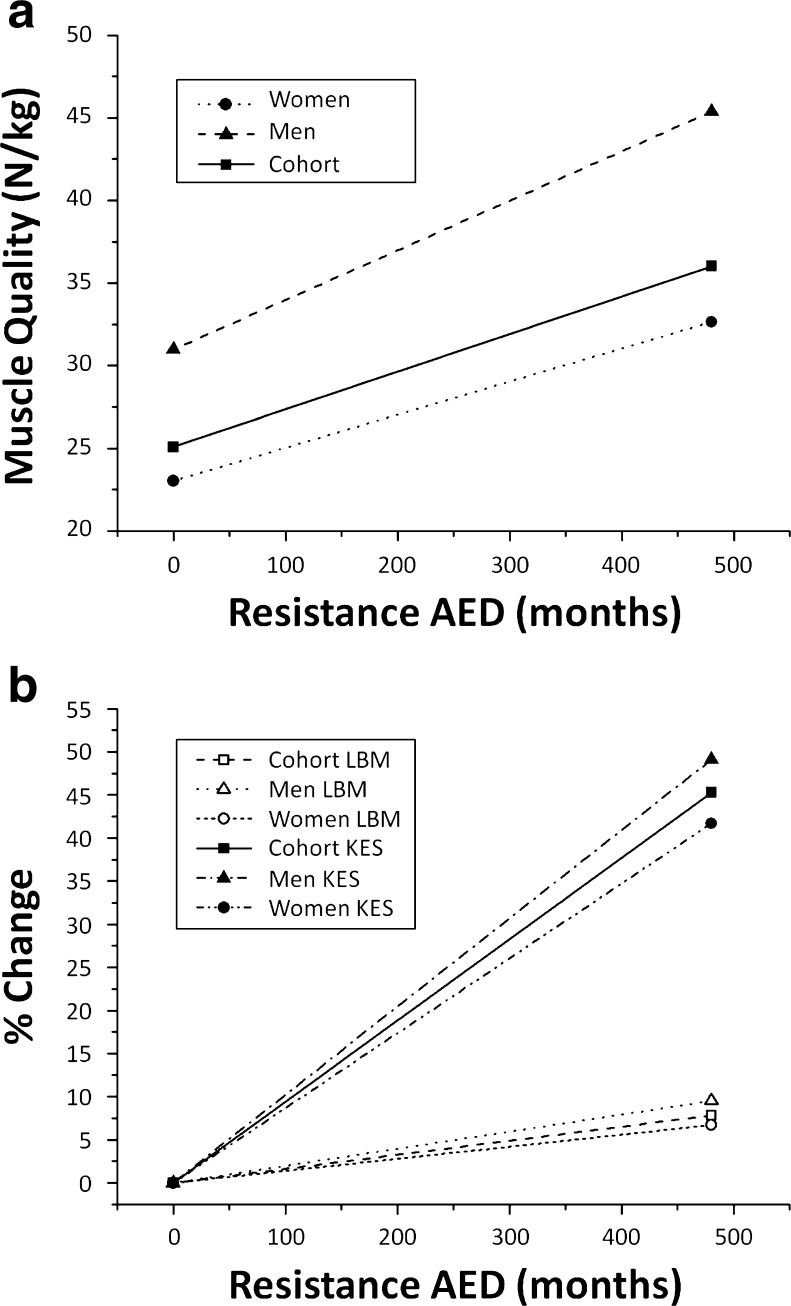
Age and gender were entered at step 1, explaining 20 % of the variance in MQ. After entry of resistance AED at step 2, the total variance explained by the model as a whole was 21.6 %, F(3, 308) = 25.410, p < 0.001. Resistance AED explained an additional 1.6 % of the variance in MQ, after controlling for age and gender (r2 change = 0.016, F change (1, 308) = 5.677, p = 0.018). In the final model, resistance AED (standardized β = 0.128; p = 0.018), age (standardized β = −0.312; p < 0.001), and gender (standardized β = 0.287; p < 0.001) predicted MQ. The relationship between resistance AED and MQ is illustrated in Fig. 1a.
Fig. 1.
Relationship between muscle mass and function with resistance AED. Curves are extrapolated from regression analyses. A maximum of 480 months was chosen to properly represent the full range of resistance AED observed in the cohort. a Relationship between muscle quality and resistance AED. Resistance AED was first included in the regression as a single predictor to generate the cohort curve. Gender and its interaction term with resistance AED were then added in the regression to generate men and women curves. b Relationship between lean body mass, muscle strength, and resistance AED. Resistance AED was first included in the regression as a single independent variable to predict right leg LBM and to generate the cohort curve. Gender and its interaction term with resistance AED were then added in the regression to generate men and women curves. Cohort, men, and women KES curves were extrapolated according to the same process. LBM and KES values are expressed in percentage of the values obtained for resistance AED = 0. Theoretical cohort, men, and women KES values for resistance AED = 0 were 212, 323, and 173 N, respectively. Theoretical cohort, men, and women LBM values for resistance AED = 0 were 8.1, 10.3, and 7.3 kg, respectively. AED average exercise duration, LBM lean body mass, KES knee extension strength
Relationship between exercise, knee extension strength, and lean body mass
First, age and gender were entered at step 1, explaining 44.6 % of the variance in KES. After entry of resistance AED at step 2, the total variance explained by the model as a whole was 46 %, F(3, 308) = 96.649, p < 0.001. Resistance AED explained an additional 1.7 % of the variance in KES, after controlling for age and gender (r2 change = 0.017, F change (1, 308) = 8.773, p = 0.003). In the final model, resistance AED (standardized β = 0.132; p = 0.003), age (standardized β = −0.324; p < 0.001), and gender (standardized β = 0.542; p < 0.001) predicted KES.
Finally, age and gender were entered at step 1, explaining 64.2 % of the variance in LBM. After entry of resistance AED at step 2, the total variance explained by the model as a whole was 64.4 %, F(3, 308) = 185.364, p < 0.001. Resistance AED explained an additional 0.1 % of the variance in LBM, after controlling for age and gender (r2 change = 0.001, F change (1, 308) = 1.140, p = 0.286). In the final model, age (standardized β = −0.233; p < 0.001) and gender (standardized β = 0.756; p < 0.001), but not resistance AED (standardized β = 0.037; p = 0.286), predicted LBM. The relationship between right lower limb KES and LBM with resistance AED is illustrated in Fig. 1b.
Influence of age in the relationship between exercise and muscle quality
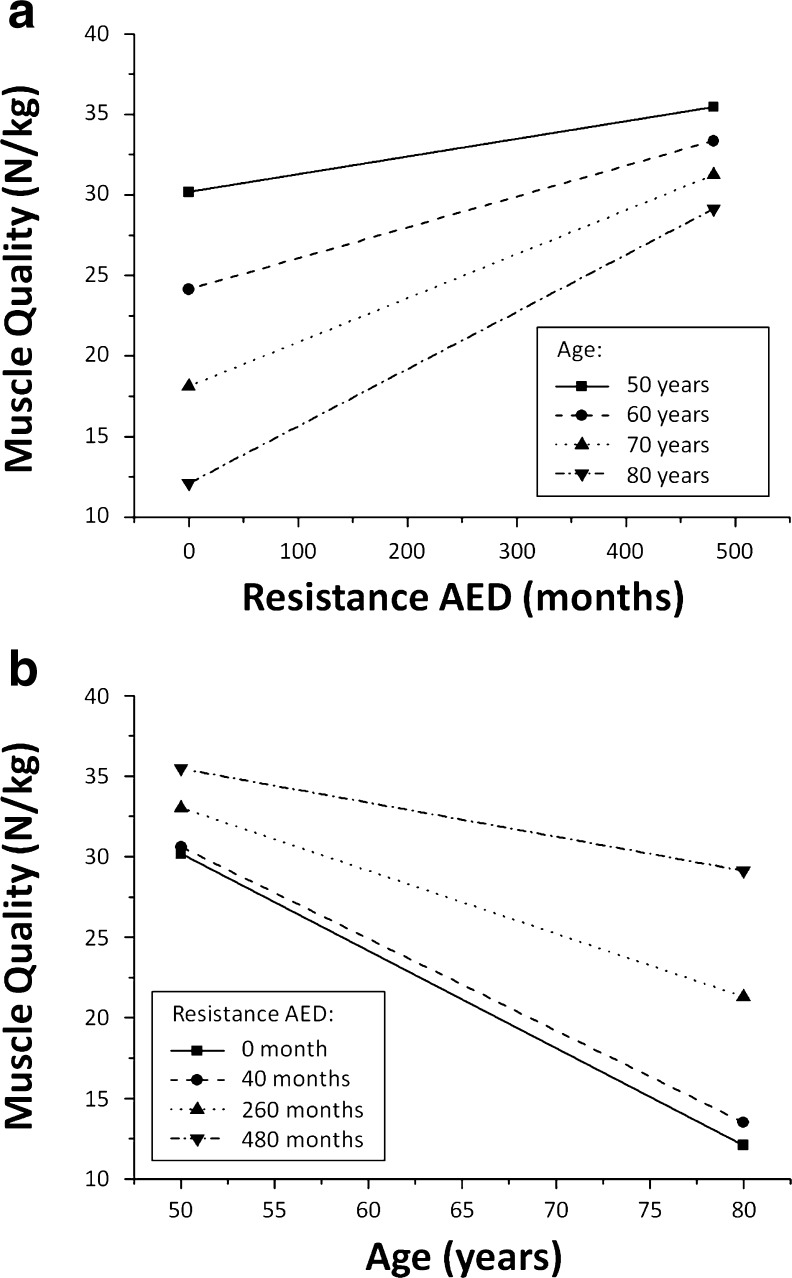
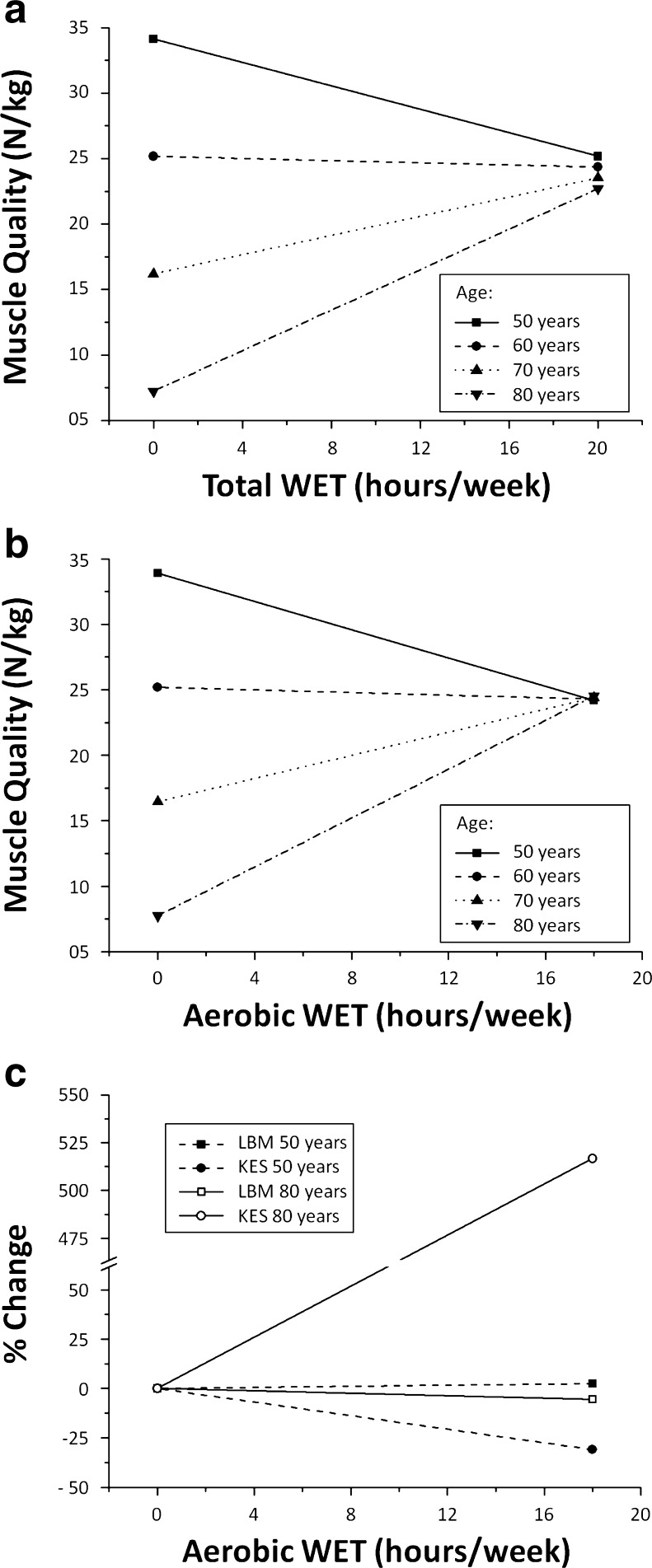
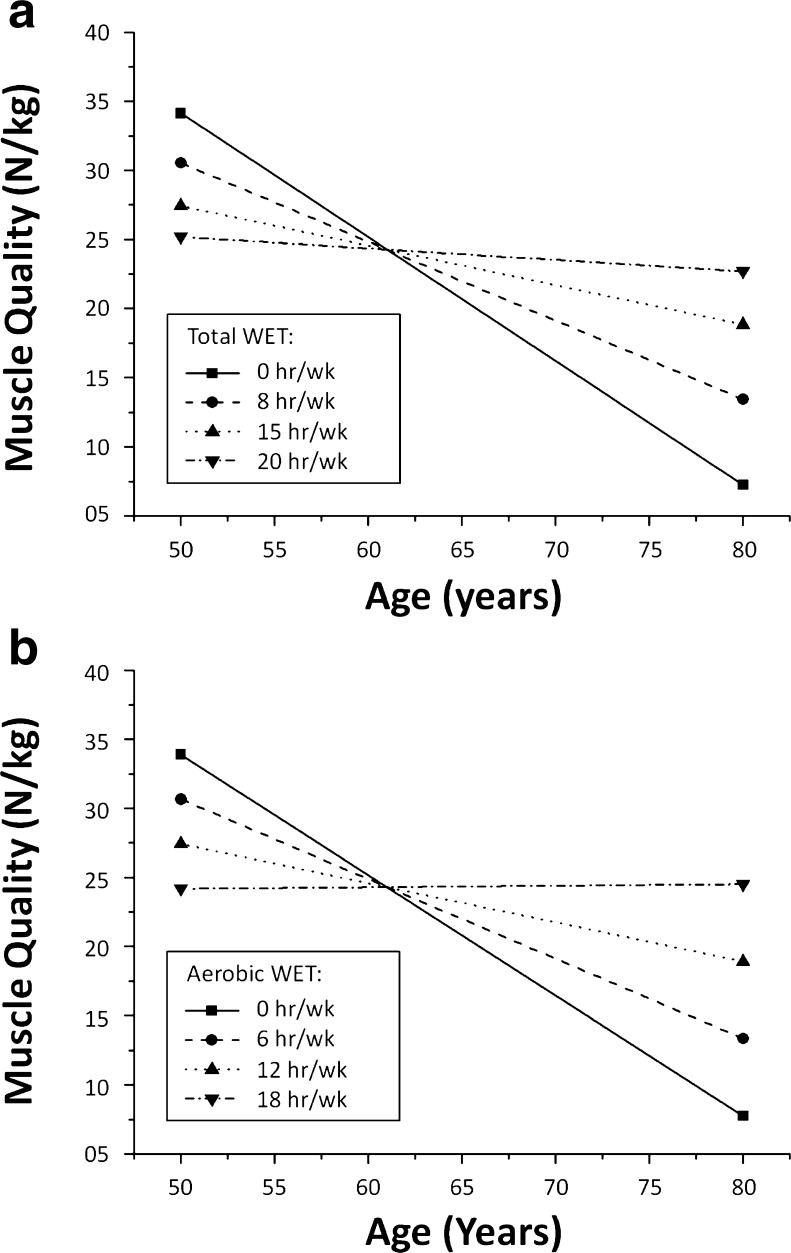
Age did not interact with resistance AED to determine MQ (standardized β = 0.035; p = 0.512). Results of the inter-relationship between age, exercise, and muscle quality are illustrated in Fig. 2a, b. Among the other exercise characteristics, only total WET (standardized β = 0.107; p = 0.046) and aerobic WET (standardized β = −0.313; p < 0.001) significantly interacted with age to determine MQ. The relationship between total WET and aerobic WET with MQ for predefined ages is illustrated in Fig. 3a–c. Figure 4a, b also illustrated the relationship between age and MQ for predefined levels of total WET and aerobic WET, respectively.
Fig. 2.
Relationship between resistance AED, age, and muscle quality. a Relationship between resistance AED and muscle quality for predefined ages. A maximum of 480 months was chosen to properly represent the full range of resistance AED observed in the cohort. b Relationship between age and muscle quality for predefined levels of resistance AED. A resistance AED of 40 months is close to the average level of the entire cohort (39.8 months). AED average exercise duration
Fig. 3.
Relationship between total and aerobic WET with muscle quality, lean body mass, and muscle strength according to age. a Relationship between total WET and muscle quality according to age. A maximum of 20 h was chosen to properly represent the full range of total WET observed in the cohort. b Relationship between aerobic WET and muscle quality according to age. A maximum of 18 h was chosen to properly represent the full range of aerobic WET observed in the cohort. c Relationship between lean body mass, muscle strength, and aerobic WET according to age. Curves are extrapolated from regression analyses in which the following were included (1) age, LBM, and their interaction term or (2) age, KES, and their interaction term. LBM and KES values are expressed in percentage of the values obtained for aerobic WET = 0. Theoretical 50- and 80-year-old individuals LBM values for aerobic WET = 0 were 7.9 and 6.3 kg, respectively. Theoretical 50- and 80-year-old individuals KES values for aerobic WET = 0 were 274 and 23 N, respectively. WET weekly exercise time, LBM lean body mass, KES knee extension strength
Fig. 4.
Relationship between age and muscle quality according to total or aerobic WET. a Relationship between age and muscle quality according to total WET. A total WET of 8 h is close to the average level of the entire cohort (8.3 h). b Relationship between age and muscle quality according to aerobic WET. An aerobic WET of 6 h is close to the average level of the entire cohort (6.2 h). WET weekly exercise time
Discussion
Several studies conducted in a laboratory-related environment have shown that exercise programs, particularly resistance training programs, had positive effects on MQ in older adults [12-15]. However, it is reasonable to question whether recreational exercise may lead to similar results.
The major finding is that resistance AED was the only factor that appears to influence muscle quality in both men and women (Fig. 1a), suggesting that practicing recreational resistance activities for a long period of time would be more important and beneficial than large amounts of varied exercises per week. When considering the cohort as a whole, resistance AED explained 1.6 % of the variance in MQ after accounting for age and gender (which explained 20 % of its variance). However, this result should be qualified since, as discussed below, age significantly influenced this relationship.
Furthermore, since muscle quality depends on both muscle mass and strength, the relationship between resistance AED and MQ may be interpreted cautiously. As illustrated in Fig. 1b, it is noteworthy that the positive association between resistance AED and MQ mainly results from a positive association between resistance AED and muscle strength. Yet, these results seem to support the recommendations for physical activity as well as the public health discourse, which state that it is beneficial to adopt a healthy lifestyle at early age and maintain it throughout the entire life to obtain benefits at older ages [24]. That is, although cross-sectional, the analyses presented here strongly suggest that lifelong exercise is beneficial for muscle function. Muscle unit survival in active elderly may be one of the factors contributing to such benefits [25].
In a recent meta-analysis, Peterson et al. [26] reported that KES increased by 33 % for a mean training duration of 18 weeks (in a laboratory-related environment) in older adults aged 50–92 years. By way of comparison, according to our results (Fig. 1b), a similar duration would be associated with strength changes of 2 %. A theoretical resistance AED of 350 months (≈29 years) would be necessary to achieve strength increases of 33 % (Note that only eight participants have exercised for that long).
Obviously, given the cross-sectional design of the present study, comparisons with the meta-analysis of Peterson et al. [26] must be examined cautiously. However, they may highlight some differences between laboratory-related and recreational exercises that could, at least partially, be attributable to differences in constraints and coaching existing between these environments. As observed by Deforche and De Bourdeaudhuij [27], while no differences were found in perceived barriers and benefits to exercise, older adults involved in supervised exercise programs had higher levels of activity and reported higher self-efficacy compared to active older adults that are not engaged in supervised exercise programs. These authors hypothesized that participation was based on their willingness to participate in a structured program, which in turn increased once more their perceptions of exercise capabilities [27]. Furthermore, there is evidence that being involved in specific programs for seniors, with instructions on how to exercise safely and with opportunities for regularly supervised activity is of importance for elderly [28]. Results of the present study suggest that the presence of a supervisor is necessary, not only for safety but also to ensure the effect of training.
The second part of the analysis dealt with the influence of age in the relationship between recreational exercises and MQ. First, it is interesting to note that age has a little influence on the relationship between resistance AED and MQ. Resistance AED was positively associated with MQ across all age groups. Furthermore, as depicted in Fig. 2a, an 80-year-old individual who have practiced resistance training exercises for 40 years would theoretically have a MQ of 2.5 times higher than that of an individual with the same age who did not train in resistance (29 and 12 N/kg, respectively). This MQ value (29 N/kg) is almost identical to the MQ of a 50-year-old individual who did not train in resistance (30 N/kg).
While bivariate tests identified resistance AED as the only predictor of MQ, interaction analyses revealed that depending on age, aerobic WET may also be taken into account (Fig. 3b). Since aerobic WET represents on average 72 % of total WET (data not shown), it is not surprising that total WET (all activities combined) also interacted with age (Fig. 3a). While aerobic WET was negatively associated with MQ in 50-year-old individuals, it was positively associated with MQ in 80-year-old participants (the transitional age being around 60 years), suggesting that aerobic training may not be overlooked in older adults. However, theoretical curves representing mean total or aerobic WET (8 and 6 h, respectively; Fig. 4) show that muscle quality is reduced by half between 50 and 80 years, although such amounts of practice are broadly superior to the recommendations of the 2011 American College of Sports Medicine (ACSM) Position Stand [24]. This suggests that recreational aerobic activities are not the preferred type of activity to maintain or increase muscle quality.
Figure 3c provides us with an explanation concerning this age-dependent relationship between aerobic WET and muscle quality. In both 50- and 80-year-old individuals, muscle mass appears to remain relatively constant, regardless of aerobic WET. However, while aerobic WET was negatively associated with muscle strength in 50-year-old individuals, it was positively associated with muscle strength in 80-year-old individuals. Yet, even the theoretical fivefold increase of the basal KES value of 80-year-old individuals who train for 18 h/week does not reach the mean KES value of a sedentary 50-year-old individual (140 vs. 270 N; see the legend in Fig. 3c).
In summary, we observed that recreational resistance AED and aerobic activities beyond 60 years were associated with MQ. It should, however, be kept in mind that other factors, such as nutrition, may be of importance. For instance, in obese individuals, the combination of diet and exercise was even more effective than exercise or diet alone to improve MQ [29, 30].
A number of limitations need to be considered. The cross-sectional design of the study does not allow us to draw conclusions as to causal associations between physical exercise, age, and muscle quality. Furthermore, physical activity was evaluated by interview, which may decrease the risk of recall bias, but increase the risk of social desirability bias. Finally, although we have statistically ensured that there was no gender interaction, it remains possible that a disproportionate number of women have influenced the results. However, the use of questionnaire allowed us to cover and identify all physical activities. The use of accurate devices to evaluate body composition and muscle quality also reinforces our findings.
Conclusion
In conclusion, our results suggest that long-term engagement in recreational exercise, especially resistance exercise, is beneficial for muscle quality and should be encouraged across all age groups. Furthermore, beyond 60 years, the duration of weekly aerobic activities also seems to be positively associated with muscle quality.
Acknowledgments
We would like to thank the YMCAs of Quebec for supporting us. SBA is supported by the Canadian Institute of Health Research (CIHR). MAL is supported by the Fonds de la Recherche en Santé du Québec (FRSQ). The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
Sébastien Barbat-Artigas, Sophie Dupontgand, Charlotte H. Pion, Yannick Feiter-Murphy, and Mylène Aubertin-Leheudre declare that they have no conflict of interest.
References
- 1.Hairi NN, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 2.Shin S, et al. Lower extremity muscle quality and gait variability in older adults. Age Ageing. 2012;41:595–9. doi: 10.1093/ageing/afs032. [DOI] [PubMed] [Google Scholar]
- 3.Barbat-Artigas S, et al. Clinical relevance of different muscle strength indexes and functional impairment in women aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2013;68:811–9. doi: 10.1093/gerona/gls254. [DOI] [PubMed] [Google Scholar]
- 4.Barbat-Artigas S, et al. Muscle quantity is not synonymous with muscle quality. J Am Med Dir Assoc. 2013;14:852.e1–7. doi: 10.1016/j.jamda.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Sipila S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14:433–42. doi: 10.1111/j.1475-097X.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard DR, Heroux M, Janssen I. Association between muscle mass, leg strength, and fat mass with physical function in older adults: influence of age and sex. J Aging Health. 2010;23:313–28. doi: 10.1177/0898264310388562. [DOI] [PubMed] [Google Scholar]
- 7.Barbat-Artigas S, et al. Exploring the role of muscle mass, obesity and age in the relationship between muscle quality and physical function. J Am Med Dir Assoc. 2014;15:303.e13–20. doi: 10.1016/j.jamda.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 10.Lindle RS, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol. 1997;83:1581–7. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, et al. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89:81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Tracy BL, et al. Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol. 1999;86:195–201. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- 13.Reeves ND, Narici MV, Maganaris CN. Effect of resistance training on skeletal muscle-specific force in elderly humans. J Appl Physiol. 2004;96:885–92. doi: 10.1152/japplphysiol.00688.2003. [DOI] [PubMed] [Google Scholar]
- 14.Cadore EL, et al. Strength prior to endurance intra-session exercise sequence optimizes neuromuscular and cardiovascular gains in elderly men. Exp Gerontol. 2011;47:164–9. doi: 10.1016/j.exger.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Radaelli R, et al. Low- and high-volume strength training induces similar neuromuscular improvements in muscle quality in elderly women. Exp Gerontol. 2013;48:710–6. doi: 10.1016/j.exger.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Kennis E, et al. Effects of fitness and vibration training on muscle quality: a 1-year postintervention follow-up in older men. Arch Phys Med Rehabil. 2013;94:910–8. doi: 10.1016/j.apmr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Chastin SF, et al. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing. 2012;41:111–4. doi: 10.1093/ageing/afr075. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill K, Reid G. Perceived barriers to physical activity by older adults. Can J Public Health. 1991;82:392–6. [PubMed] [Google Scholar]
- 19.Hui EK, Rubenstein LZ. Promoting physical activity and exercise in older adults. J Am Med Dir Assoc. 2006;7:310–4. doi: 10.1016/j.jamda.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39:1056–61. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 23.Stevens JA, et al. Gender differences in seeking care for falls in the aged Medicare population. Am J Prev Med. 2012;43:59–62. doi: 10.1016/j.amepre.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Garber CE, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 25.Power GA, et al. Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc. 2010;42:1644–50. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- 26.Peterson MD, et al. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–37. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deforche B, De Bourdeaudhuij I. Differences in psychosocial determinants of physical activity in older adults participating in organised versus non-organised activities. J Sports Med Phys Fitness. 2000;40:362–72. [PubMed] [Google Scholar]
- 28.Sidney KH, Shephard RJ. Attitudes towards health and physical activity in the elderly. Effects of a physical training program. Med Sci Sports. 1976;8:246–52. [PubMed] [Google Scholar]
- 29.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–9. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villareal DT, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]