Fig. 2.
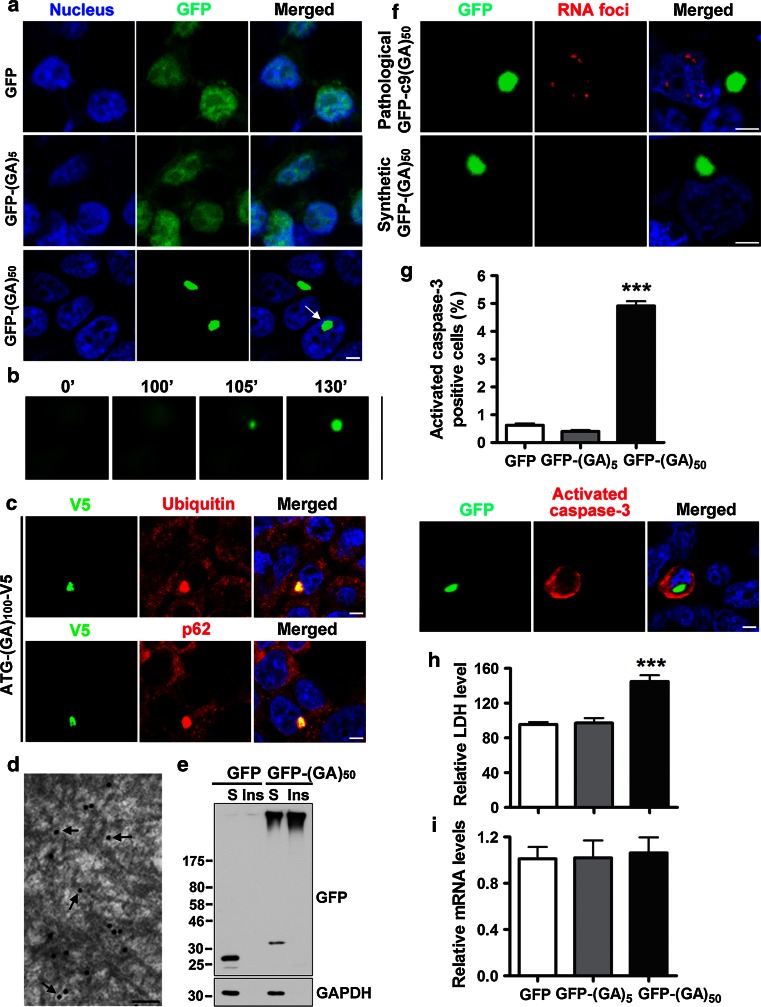
Poly(GA) proteins form inclusions and are toxic in cultured cells. a Expression of GFP-(GA)50 in cultured HEK293T cells results in the formation of cytoplasmic or nuclear (arrow) inclusions. Scale bar represents 5 µm. b Representative images of live cells demonstrating how quickly inclusions form (compare the image at 100 min to the image at 105 min). c Poly(GA) inclusions are ubiquitin and p62-positive in cultured cells. Scale bar represents 5 µm. d Immuno-electron microscopy with an anti-GFP antibody labeled with gold particles shows that cytoplasmic GFP-(GA)50 inclusions are composed of filamentous structures (arrow). Scale bar represents 100 nm. e Western blot analysis of Triton X-100 soluble (S) and insoluble (Ins) cell lysates shows that a portion of poly(GA) proteins forms high molecular weight material. f Cultured cells were made to express GFP-(GA)50, which encodes a synthetic repeat sequence (GGXGCX)50 (where X represents a random nucleotide), or GFP-c9(GA)50, in which the pathological repeat sequence (GGGGCC)50 was used. Post-transfection, cells were subjected to RNA fluorescence in situ hybridization (FISH) to visualize RNA foci. GFP-(GA)50 expression leads to the formation of poly(GA) inclusions, but transcripts from this sequence do not form RNA foci. In contrast, both RNA foci and poly(GA) inclusions are formed in cells that express GFP-c9(GA)50. Scale bar represents 5 µm. g Quantitative analysis and representative image showing that cells bearing inclusions of GFP-(GA)50 are immunoreactive for activated caspase-3, a crucial mediator of cell death. Scale bar represents 5 µm. h LDH activity in media, an indicator of cell toxicity, is increased in cells expressing GFP-(GA)50. i Transgene mRNA levels are comparable among cells expressing GFP, GFP-(GA)5 and GFP-(GA)50. Data represent the mean ± SEM from sixteen random selected fields (g) or three separate experiments (h and i). ***P < 0.001, as analyzed by one-way analysis of variance followed by Tukey’s post hoc analysis