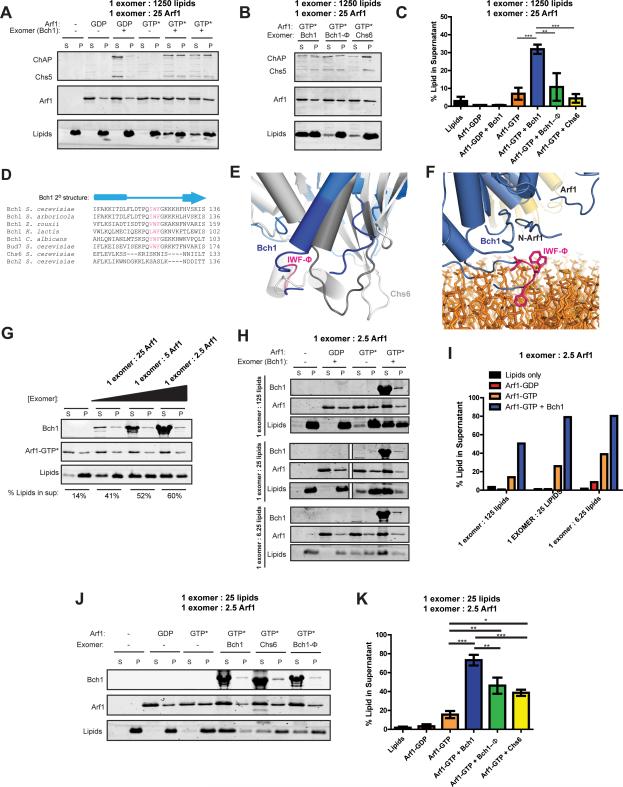
Figure 6. Exomer amplifies Arf1 membrane remodeling activity.
A) Liposome vesiculation assay to examine membrane remodeling by Arf1 and exomer. Substrate liposomes sediment to the pellet (P). Smaller liposomes generated by membrane fission remain in the supernatant (S). The Chs5(1-299)/Bch1 exomer complex was used in these experiments. GTP* = GMPPNP. Molar ratios of components are indicated.
B) Similar to (A), but comparing Chs5(1-299)/Bch1, the hydrophobic mutation in Chs5(1-299)/Bch1 (Φ), and Chs5(1-299)/Chs6.
C) Quantification of vesiculation in (A) and (B). n = 3.
D) Multiple sequence alignment showing conservation and secondary structure of the region surrounding the Bch1 hydrophobic element. The IWF motif sequence is conserved in Bch1 across multiple species as well as with the homologous ChAP Bud7. The more distantly related ChAPs (Bch2 and Chs6) do not possess this motif.
E) Superposition of the membrane proximal regions of Chs6 (gray) and Bch1 (blue). Residues included in the multiple sequence alignment in (E) are darkened. The hydrophobic membrane insertion motif is colored pink. This loop in Bch1 inhabits a position occupied by two helices that are present in Chs6 but absent from the Bch1 protein. This loop is flexible in solution, as its electron density is visible in only one of four copies in the ASU.
F) Structural view of the Bch1 hydrophobic element modeled adjacent to a membrane. Bch1 is colored blue, with the IWF sequence colored pink. Lipids are colored orange and Arf1 is yellow. The N-terminus of ΔN17-Arf1 is noted.
G) Titration of Chs5/Bch1 exomer complex in the vesiculation reactions. At the highest exomer concentration, the exomer:lipid ratio is 1:25. Quantification is noted at the bottom of the gel.
H) Titration of lipids in the vesiculation reactions.
I) Quantification of (H).
J) Similar to (A) and (B), with higher protein:lipid ratio.
K) Quantification of (J).
See also Figure S4.