Abstract
Retinal Müller glial cells (RMGs) have a primary role in maintaining the homeostasis of the retina. In pathological situations, RMGs execute protective and regenerative effects, but they can also contribute to neurodegeneration. It has recently been recognized that cultured primary RMGs secrete pro-survival factors for retinal neurons for up to 2 weeks in culture, but this ability is lost when RMGs are cultivated for longer durations. In our study, we investigated RMG supernatants for novel neuroprotective factors using a quantitative proteomic approach. Stable isotope labeling by amino acids in cell culture (SILAC) was used on primary porcine RMGs. Supernatants of RMGs cultivated for 2 weeks were compared with supernatants from cells that had already lost their protective capacity. Using this approach, we detected established neurotrophic factors such as transferrin, osteopontin, and leukemia inhibitory factor and identified C-X-C motif chemokine 10 (CXCL10) as a novel candidate neuroprotective factor. All factors prolonged photoreceptor survival in vitro. Ex vivo treatment of retinal explants with leukemia inhibitory factor or CXCL10 demonstrated a neuroprotective effect on photoreceptors. Western blots on CXCL10- and leukemia inhibitory factor–stimulated explanted retina and photoreceptor lysates indicated activation of pro-survival signal transducer and activator of transcription signaling and B-cell lymphoma pathways. These findings suggest that CXCL10 contributes to the supportive potential of RMGs toward retinal neurons.
The vascularized mammalian retina contains three types of glial cells: microglia, astrocytes, and retinal Müller glial cells (RMGs)1 (1, 2). RMGs span the entire depth of the retina and therefore constitute an anatomical link between all retinal neurons and the compartments needed for the exchange of molecules, such as blood vessels, the vitreous body, and the subretinal space (3, 4). Significantly involved in the organization of the developing retina (5), RMGs have diverse functions in the adult retina, such as the proposed “metabolic symbiosis” with retinal neurons (3, 6), neurotransmitter recycling (7), and control of retinal K+ and water homeostasis (3, 8). Destruction of RMGs causes retinal dysplasia, photoreceptor (PR) apoptosis, and retinal degeneration, demonstrating their importance for the maintenance of retinal structure and function (3, 4). Most important, RMGs produce a so-called gliotic phenotype in response to a large variety of retinal diseases, which involves the up-regulation of filaments, dedifferentiation accompanied by loss of physiologic function, and occasionally proliferation (4). Under physiological conditions, RMGs provide neurotrophic survival cues for retinal neurons, especially photoreceptors (4). Although some RMG-derived neurotrophic factors have been identified in targeted analyses (9) or transcriptome studies (10), a comprehensive understanding of the RMG secretome is still lacking. The identification of survival molecules for retinal neurons could be particularly beneficial for degenerative retinal conditions such as age-related macular degeneration or retinitis pigmentosa, a family of diseases in which the death of retinal photoreceptors leads to irreversible blindness. Efforts have been undertaken to characterize the efficacy of well-known neurotrophic factors against retinal degeneration (11).
We have previously established an in vitro model for studying primary RMG secretomes, demonstrating that these cells secrete a mixture of proteins that functionally prolong the survival of primary photoreceptors (PRs) (12). However, primary RMGs trans-differentiate during in vitro culture (13), resulting in the loss of PR-promoting survival properties (12). We performed an mRNA-based screening to compare neuroprotective and trans-differentiated RMG phenotypes, and we found that very few transcripts were altered between RMGs that had survival-promoting properties (day 14 cells) and those that had lost this activity (day 21 cells) (12). Unfortunately, the proteins related to the changed transcripts did not provide positive survival cues for PRs (data not shown). As our transcriptome screen did not reveal the identity of the RMG-derived molecules present in the secretomes, we established stable isotope labeling by amino acids in cell culture (SILAC) with primary RMGs. The cells were sufficiently labeled following 3 weeks in culture (day 21 cells), and we detected changes in the protein expression of cellular lysates that were indicative of trans-differentiation toward a fibroblast-like phenotype between day 14 and day 21 (13).
Using this model, we conducted a quantitative protein expression screen to examine differences in the RMG secretome, and we identified several potential neuroprotective molecules that correlated with the functional survival supporting phenotype. Along with previously established neurotrophic factors osteopontin (SPP1) (10), leukemia inhibitory factor (LIF) (14), and the iron-stress protective receptor transferrin (15, 16), we found the novel RMG-derived molecule C-X-C motif chemokine 10 (CXCL10) (previously known as IP-10). We examined the neurotrophic activity of transferrin, LIF, and CXCL10 on isolated primary PRs, and LIF and CXCL10 were further validated on retinal explants from a mouse model of retinal degeneration. Western blots of explanted porcine retina and porcine PR lysates indicated the activation of survival-prolonging signal transduction upon stimulation with CXLC10 or LIF. SILAC-based screening of a primary RMG model system therefore enabled the characterization of a mixture of neurotrophic factors secreted by RMGs.
EXPERIMENTAL PROCEDURES
Preparation and Culture of RMGs
Porcine RMGs were prepared as previously described (12). Major blood vessels were removed, and the retina was cut into smaller segments and then washed twice in Ringer's solution. Retinal tissue was dissociated by treatment with activated papain (Worthington) followed by a DNase (Sigma) digest and gentle trituration using a fire-polished Pasteur pipette. Dissociated cells were collected by centrifugation at 240 × g for 5 min and resuspended in SILAC-DMEM medium (PAA, GE Healthcare, Munich, Germany) supplemented with 0.23 mg/ml proline (Silantes, Munich, Germany), 4 mm l-glutamine (PAA), 10% dialyzed fetal bovine serum (FBS) (PAA), 50 units/ml penicillin (Invitrogen, Heidelberg, Germany), and 0.05 mg/ml streptomycin (Invitrogen). 0.1 mg/ml 13C615N2-l-lysine and 0.1 mg/ml 13C615N4-l-arginine (Silantes) were added to the “heavy” medium, and normal amino acids (Silantes) to the “light” medium. Cells were plated onto 10-cm-diameter cell culture dishes (Nunc, Thermo Scientific, Dreieich, Germany) and incubated overnight at 37 °C. The following day, non-attached cells were removed by gentle panning, and the remaining RMG cells were cultured for 14 or 21 days to near confluence, with the media changed every 4 to 7 days. The day before harvesting, RMGs were washed three times with PBS and cultivated in FBS-free medium for 3 h. The medium was replaced once more, and cells were starved for another 18 h. A total of three SILAC experiments were performed with day 21 conditions heavy labeled.
Preparation of RMG Supernatants
After starvation, culture supernatants were collected, filtered (0.22 μm) to remove large cellular debris, and stored at −80 °C. The total protein content of RMG supernatants was measured using the Nanoquant protein assay (Roth, Karlsruhe, Germany) with concentrations ranging from 8 ng/μl to 14 ng/μl per sample. For every SILAC experiment, 40 μg of the respective day 14 and day 21 samples were mixed at a 1:1 protein ratio before precipitation with ice-cold acetone at a ratio of 1:5 at −20 °C overnight. Protein pellets were dissolved in urea buffer consisting of 4 m urea, 2 m thiourea, 4% CHAPS, and 4 mm DTT and prefractionated via one-dimensional SDS-PAGE.
Prefractionation and Digestion of Samples
A total of 80 μg per combined SILAC sample pair was loaded per lane on a 12% one-dimensional SDS-PAGE gel, and proteins were separated for ∼5 cm. The gel was fixed and stained using Coomassie dye (0.1% (w/v) Coomassie Brilliant Blue R-250 in 50% (v/v) methanol, 10% (v/v) acetic acid). Each lane was excised, fractionated into six bands for the supernatants, and subjected to in-gel digestion as previously described (13).
Mass Spectrometry
Peptide samples were dissolved in 2% acetonitrile/0.5% trifluoroacetic acid by means of agitation and incubation for 30 min at room temperature. Before loading, the samples were centrifuged for 5 min at 4 °C. LC-MS/MS analysis was performed as previously described (17). Every sample was automatically injected and loaded onto the trap column at a flow rate of 30 μl/min in 5% buffer B (98% acetonitrile/0.1% formic acid in HPLC-grade water) and 95% buffer A (2% acetonitrile/0.1% formic acid in HPLC-grade water). After 5 min, the peptides were eluted from the trap column and separated on the analytical column by a 120-min gradient from 5% to 31% buffer B at 300 nl/min followed by a short gradient from 31% to 95% buffer B in 5 min. Between each sample, the gradient was set back to 5% buffer B and left to equilibrate for 20 min. From the MS prescan, the 10 most abundant peptide ions were selected for fragmentation in the linear ion trap if they exceeded an intensity of at least 200 counts and if they were at least doubly charged. During fragment analysis, a high-resolution (60,000 full width at half-maximum) MS spectrum was acquired in the Orbitrap with a mass range from 200 to 1500 Da.
SILAC Analysis
Sample compositions of the six supernatant fractions of each of the three independent SILAC experiments were analyzed using MaxQuant software (version 1.2.0.25, Max Planck Institute of Biochemistry, Martinsried, Germany) (18) with its internal search engine Andromeda (19). Default search parameters were used, allowing two missed cleavages, a fragment ion mass tolerance of 0.5 Da, and a parent ion tolerance of 20 ppm. Carbamidomethylation was set as a fixed modification; methionine oxidation and N-terminal acetylation were allowed as variable modifications. Labeling was set to doublets (0/0 und 8/10). The Andromeda search engine was configured for the Ensembl Pig protein database (version 62, 8,747,138 residues, 19,083 sequences) including a decoy database as well as a common-contaminants database. The MaxQuant output was filtered for reverse identifications, contaminants, findings “only identified by site,” and findings identified with more than one peptide. For analysis of the supernatants, only candidates identified in a least two out of three experiments were pursued. Neurotrophic candidates were selected based on the fold change with the following criteria: a minimum of 10 SILAC pairs per quantification, a maximum SILAC-pair variability of 100%, and a maximum standard deviation between the fold changes of 50% per protein across experiments. 30 candidates with a 2-fold greater abundance at day 14 were loaded into Ingenuity Pathway Analysis (INGENUITY System) (20), and only candidates with an extracellular localization were pursued further.
Photoreceptor Preparation and Survival Assay
Porcine PRs were prepared and cultured as described elsewhere (12). Human recombinant CXCL10 (100 ng/ml; Prospec, Rechovot, Israel), human Holo-transferrin (1000 ng/ml; Prospec), human recombinant LIF (1 and 100 ng/ml; Prospec), human recombinant CCL5 (2 and 10 ng/ml; Peprotec, Hamburg, Germany), and human recombinant IL8 (10 and 100 ng/ml; Peprotec) in DMEM/F-12 medium (Invitrogen) or medium alone (negative control) were applied to the PR cultures 20 h after preparation. PR survival was monitored via an esterase calcein-fluorophore assay (Molecular Probes, Darmstadt, Germany) in a 96-well assay format. Total fluorescence per well is linearly correlated to the number of living cells per well (12) and was measured daily for 6 days using a fluorescence reader (Synergy HT, BioTek, Bad Friedrichshall, Germany) and compared with the initial fluorescence. All PR survival assay experiments, with the exception of 100 ng/ml LIF treatment, were performed at least three times.
Retinal Explants
Retinal explant experiments were performed using retinas from C3H/HeH mice carrying the Pde6brd1 mutation. Animals were bred and maintained in ventilated cages at 22 °C ± 1 °C with a 12-h light/dark cycle and free access to water and food. All animals in this study were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Long-term Mouse Organotypic Explants
Retinas were isolated from 5-day-old mice, with the day of birth defined as postnatal day 0, with the retinal pigment epithelium attached as described previously (10, 21). Enucleated eyes were incubated at 37 °C for 15 min in R16 serum-free medium (Invitrogen) containing 0.12% proteinase K (MP Biomedicals, Santa Ana, CA). The enzymatic digestion was stopped by the addition of 10% FBS (Invitrogen); the anterior segment, lens, vitreous, sclera, and choroid were carefully removed, and the retina was dissected free. The retina was cut perpendicular to its edges, resulting in a coverleaf-like shape, and transferred to a 0.4-μm polycarbonate membrane insert (Costar, Fisher Scientific, Schwerte, Germany) with the retinal pigment epithelium directly facing the membrane. The insert was placed into a six-well culture plate and incubated at 37 °C in enriched R16 (Invitrogen) nutrient medium (22), which was replaced every second day. After 2 days in culture, the explants were treated with 100 ng/ml CXCL10 (R&D Systems, Wiesbaden, Germany), 50 ng/ml LIF (Prospec), or a combination of 100 ng/ml CXCL10 and 50 ng/ml LIF (or kept as an untreated control) and then cultured for an additional 12 days. The explants were fixed in 4% paraformaldehyde and cryoprotected by incubation in a sucrose gradient. Explants were embedded in Tissue Tek (Sakura, Alphen aan den Rijn, The Netherlands), and transverse 14-μm sections were cut on a cryotome (CM 1850, Leica, Wetzlar, Germany). All sections were collected from tissue close to the optic nerve head. To monitor the consequences of treatment, retinal sections were defrosted, washed with 0.1 m PBS, stained with 4′,6-diamidino-2-phenylindole, washed again, and coverslipped. The sections were photographed with an AxioCam HRC (Carl Zeiss, Jena, Germany) mounted on an Axioskop 2 microscope (Carl Zeiss) using a Plan Neofluor Ocular with ×20 magnification. The thickness of the outer nuclear layer (ONL) was determined from the micrographs using Image J software (version 1.44p, NIH) at five different positions on each section and in a total of 25 sections per explant.
Stimulation of Organotypic Explant Porcine Retinas
Porcine eyes were treated as described in Refs. 10 and 12. Briefly, enucleated eyes were first cleaned of periocular tissues and dissected along the ora serata. Next, the retina and the vitreous were removed, and the retina was separated from the vitreous and transferred to a Petri dish containing R16 serum-free medium. The retina was cut perpendicular to its edges to appropriate pieces of ∼1 cm2 and transferred to a 0.4-μm polycarbonate membrane insert (Costar) with the photoreceptor side directly facing the membrane. The insert was placed into a six-well culture plate and incubated in R16 nutrient-enriched medium at 37 °C. For stimulation experiments, retinas were incubated in media containing LIF (50 ng/ml) or CXCL10 (100 ng/ml) for 24 h. The factors were absent from the media in control experiments. After 24 h, the retinal tissue was directly lysed and used for Western blot analyses.
Immunofluorescence
CXCL10 expression was examined with immunofluorescence in porcine retina. Porcine eyes were dissected with the lens, cornea, and vitreous removed. The posterior eyecup was fixed in 4% paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.4) and cryoprotected in graded sucrose (10%, 20%, 30%) (23). Vertical 12-μm cryosections were blocked with 10% normal goat serum, 1% BSA, and 0.5% Triton-X 100 in phosphate buffer for 1 h and incubated at room temperature overnight either with anti-CXCL10 antibody (orb10277, Biorbyt, Cambridge, UK) diluted 1:200 in buffer (3% normal goat serum, 1% BSA, and 0.5% Triton-X 100 in phosphate buffer) or in antibody buffer only. After rinsing, sections were incubated with goat anti-rabbit pig Alexa Fluor 488 secondary antibody (Invitrogen) diluted 1:1000 in buffer for 90 min, washed in phosphate buffer, and coverslipped. All sections were photographed with constant variables on an AxioImager Z1 with an ApoTome attachment (Zeiss).
Western Blot Analysis
Western blot analysis was used to determine the protein levels of phosphorylated STAT3 (pSTAT3) and BCL-2 from 20 μg of total cell lysates with α-tubulin as the loading control. For Western blot analysis, PRs were prepared as described in Refs. 10 and 12 using a pool of 20 eyes per experiment to ensure enough material. After cultivation overnight, PRs were starved for three hours and stimulated using human recombinant CXCL10 (1000 ng/ml, Prospec) or human recombinant LIF (100 ng/ml, Prospec) or left in starving medium for four hours. Blots were incubated overnight with the following primary antibodies: rabbit anti-pSTAT3 (1:7000; #9145, Cell Signaling (Leiden, The Netherlands), BCL-2 (1:1000; B3170, Sigma, Munich, Germany) or α-tubulin (1:10,000; ab6160, Abcam, Cambridge, United Kingdom). Appropriate secondary horseradish-peroxidase-coupled antibodies were used in a dilution of 1:10,000. Protein signals were visualized using the ECL Plus enhanced chemiluminescence kit (GE Healthcare, Munich, Germany). Semi-quantitative analysis of intensities was performed using ImageJ software.
Data Analysis
Data were evaluated using Prism software (version 6, GraphPad) and Excel 2010 (Microsoft). For Table I, fold changes between 0 and 1 were transformed according to the formula Neg Fc = −1 × Fc−1 to achieve negative values. The photoreceptor survival assays were analyzed using Student's t test. For explants, a nonparametric analysis of variance was performed comparing the experimental groups with the Kruskal–Wallis test followed by Dunn's post-test, correcting for multiple testing. p values less than 0.05 were considered to indicate statistically significant differences.
Table I. Neurotrophic candidates.
| Ensembl I.D. | Ratio d14/d21 | Gene | Description | Location | Family |
|---|---|---|---|---|---|
| ENSSSCP00000009579 | 24.9 | CXCL10 | Chemokine (C-X-C motif) ligand 10 | Extracellular space | Cytokine |
| ENSSSCP00000000717 | 12.4 | C1R | Complement component 1, r subcomponent | Extracellular space | Peptidase |
| ENSSSCP00000010668 | 10.2 | LIF | Leukemia inhibitory factor | Extracellular space | Cytokine |
| ENSSSCP00000012407 | 9.1 | TF | Transferrin | Extracellular space | Transporter |
| ENSSSCP00000010537 | 9.0 | PLBD2 | Phospholipase B domain containing 2 | Extracellular space | Other |
| ENSSSCP00000001528 | 6.5 | C4A/C4B | Complement component 4B | Extracellular space | Other |
| ENSSSCP00000000719 | 3.7 | C1S | Complement component 1, s subcomponent | Extracellular space | Peptidase |
| ENSSSCP00000009825 | 3.2 | SPP1 | Secreted phosphoprotein 1 | Extracellular space | Cytokine |
| ENSSSCP00000002564 | 2.7 | NPC2 | Niemann-Pick disease, type C2 | Extracellular space | Other |
| ENSSSCP00000016249 | 2.3 | TFPI2 | Tissue factor pathway inhibitor 2 | Extracellular space | Other |
| ENSSSCP00000014011 | 2.1 | SERPING1 | Serpin peptidase inhibitor, clade G, member 1 | Extracellular space | Other |
RESULTS
RMG culture supernatants are known to contain neurotrophic factors (24–26), and a previous study demonstrated the survival-prolonging effect of porcine RMG supernatants on photoreceptors in vitro (12). In this study, we used a quantitative mass spectrometric approach on primary RMGs to identify currently unknown neurotrophic factors (Fig. 1).
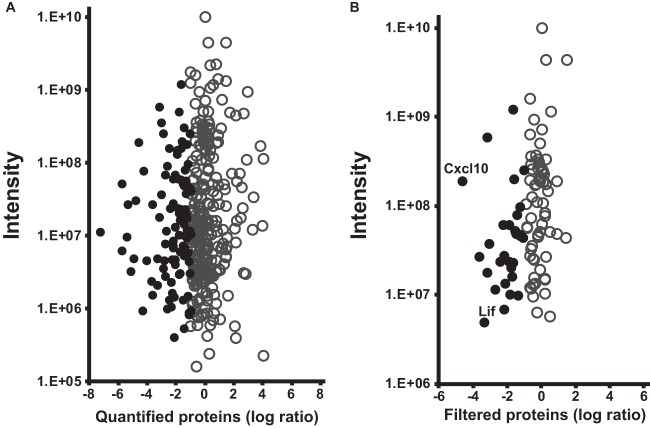
Fig. 1.
Identification of neurotrophic candidate proteins using SILAC quantification. A total of 376 protein groups were reliably identified in the RMG supernatants. A, protein abundances are displayed as log transformed heavy-to-light ratios between cells cultured for 21 days and cells cultured for 14 days, independent of their intensity. Negative ratio values represent a greater abundance of proteins on day 14 than on day 21 (black dots). B, for the identification of neurotrophic candidates, stringent filtering criteria were applied to proteins with differential abundances with respect to quality of quantification, secretion, and fold change.
Labeling Efficiency of Primary Porcine RMGs Using the SILAC Approach
Sufficient labeling is a prerequisite for reliable quantifications using SILAC and requires the use of dialyzed FBS as a supplement to cell culture medium to avoid all sources of nonlabeled amino acids. The ability of primary RMGs cultivated under SILAC conditions to produce neurotrophic factors was examined and confirmed using PR survival assays (Fig. 2). In order to control for labeling efficiency, our primary cells grown under SILAC heavy condition were analyzed as described (13). Cells cultured for 21 days reached a median labeling efficiency of more than 90% (supplemental Fig. S1), which is in accord with other studies using primary cells (27, 28). As primary RMGs cultured for 14 days reached a median labeling of only 50% (supplemental Fig. S1), no reverse SILAC experiment (heavy labeling of day 14 primary cells) was performed. All three SILAC experiments were therefore performed with day 21 cells grown under SILAC heavy conditions compared with day 14 cells grown in light (unlabeled) medium.
Fig. 2.

Transferrin, LIF, and CXCL10 directly promote PR survival in vitro. Isolated and cultured porcine PRs were used to assess the neurotrophic capacities of either RMG-conditioned medium or the individual candidate neurotrophic factors identified via the quantitative SILAC approach. PRs were treated with supernatant from day 14 or day 21 cultured RMGs or medium containing neurotrophic candidates transferrin (1000 ng/ml transferrin), LIF (1 ng/ml), or CXCL10 (100 ng/ml). After 6 days in vitro, the day 14 supernatants and all three candidates were found to significantly enhance PR survival relative to controls and day 21 supernatant (t test p < 0.05). FC, fold change.
Differential Quantification of RMG Supernatants Identifies Neurotrophic Candidate Proteins
A total of 376 protein groups were reliably identified in the RMG supernatants (Fig. 1A). Protein abundances are displayed as log transformed heavy-to-light ratios between cells grown for 21 days and 14 days, independent of their intensity. Negative ratio values represent a greater abundance of protein on day 14, and therefore these proteins are considered potential neurotrophic candidates (black circles). Proteins with a greater abundance at day 14 were further filtered to ensure solid quantification as well as secretion. Accuracy of the quantification was ensured by employing a minimum of 10 SILAC pairs per quantification. Candidates had to be identified in a least two out of three experiments, and a maximum of 100% SILAC pair variability was allowed. This value takes into account the high diversity of material (primary cells from different animals that were not inbred or age- or sex-matched) and the fact that ratios at the extremes vary to a higher degree but come from the potentially most interesting candidates. To exclude proteins that did not demonstrate a coherent expression pattern, we allowed a maximum standard deviation of 50% per protein group between the ratios of the experiments (Fig. 1B). Finally, 30 proteins (supplemental Table S1) demonstrating at least a 2-fold greater abundance at day 14 were tested for their cellular localization to discriminate between secreted proteins and cell leakage or breakage. Table I summarizes the 11 potential neurotrophic candidate proteins that were assigned to the extracellular space.
Among the most differentially expressed proteins between the experimental groups were the known neurotrophic factors SPP1 (10) and LIF (29). The extracellular receptor transferrin demonstrated an almost 10-fold higher expression on day 14 and has been reported to play a role in photoreceptor survival (16). Our quantitative approach identified not only known neurotrophic factors, but also a novel candidate that has yet to be discussed in a neuroprotective context. The chemokine CXCL10 exhibited the greatest fold change between days 14 and 21 of all our identified proteins (Fig. 1B). Based on these results, the following analyses focused on the cytokine candidates, SPP1, LIF, CXCL10, and the iron receptor transferrin.
Survival Assays on Primary Porcine Photoreceptors Confirm Neurotrophic Activity of Candidates
Photoreceptor survival assays were performed to validate the neuroprotective potential of transferrin, LIF, and CXCL10. To test the validity of our filtering approach, chemokine ligand 5 (CCL5) and interleukin 8 (IL8) were also tested, as they were more abundant on day 14 but did not meet all filtering criteria. Survival of PRs was monitored by a calcein-esterase assay (12). After 6 days in vitro, transferrin, LIF, and CXCL10 significantly enhanced PR survival (Fig. 2). Neither CCL5 nor IL8 enhanced PR survival relative to controls (supplemental Fig. S2).
CXCL10 Is Expressed in Porcine Retina in Vivo
We addressed the question of whether CXCL10 is also present in healthy retina or induced by culture conditions. First, expression of CXCL10 was confirmed on total retinal tissue by means of RT-PCR (data not shown). To gain more insight on the CXCL10 distribution and the cells of origin, an immunofluorescence assay was conducted on porcine retina.
We found intense and specific CXCL10 labeling within the ganglion cell layer and some amacrine cells of the inner nuclear layer, in addition to weak labeling of other cells in the inner nuclear layer and diffuse fluorescent labeling throughout the retina, which is expected for a secreted protein (Fig. 3A). This confirmed CXCL10 in vivo expression in the retina and suggests that CXCL10 is produced in vivo by several cell types.
Fig. 3.
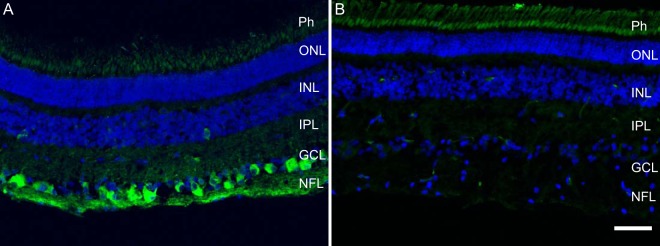
CXCL10 expressed in porcine retina in vivo. Fluorescent labeling of CXCL10 was diffusely present throughout the retina (A) and was particularly intense in the cells of the ganglion cell layer and some amacrine cells in the inner nuclear layer. In comparison, only background fluorescence was present in the photoreceptors in the negative control (B). Scale bar: 50 μm. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
LIF and CXCL10 Decrease Cell Death in Pde6brd1 Retina Explants
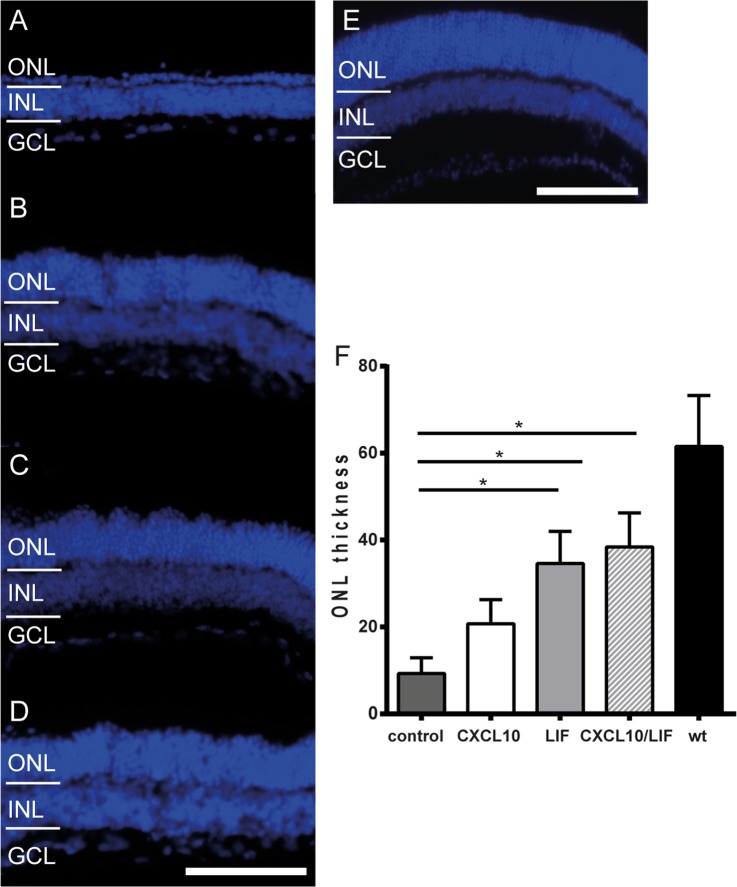
To assess whether the neurotrophic effects of LIF and CXCL10 counteracted PR degeneration, we conducted ex vivo retina explant cultures to monitor cell survival in intact tissue over extended periods of time (10, 30). Retinal explant cultures were prepared from C3H/HeJ mice, which carry the Pde6brd1 mutation resulting in fast-degenerating PRs (31). Retinas were explanted at postnatal day 5 and cultured for 14 days with or without LIF or CXCL10, and the ONL, where PR cell bodies are located, was measured. Fig. 4 shows photomicrographs of explanted Pde6brd1 retinas and a freshly dissected wild-type C57/Bl6 retina. The ONLs of the Pde6brd1 untreated retinas displayed only single layers of nuclei in the ONL (Fig. 4A), whereas the LIF-treated (Fig. 4B), the CXCL10-treated (Fig. 4C), and the combined CXCL10/LIF-treated (Fig. 4D) retinas showed comparatively increased ONL size. None of the treatments, however, led to wild-type ONL thickness (Figs. 4E and 4F). Statistical analysis indicated that retinas treated with LIF (35 μm ± 7.3), CXCL10 (20 μm ± 5.5), or the combined treatment (38 μm ± 7.9) exhibited significantly thicker ONLs than untreated Pde6brd1 retinas (9 μm ± 3.9).
Fig. 4.
LIF and CXCL10 showed protective effects in ex vivo retina explants. Retinas from mice with the Pde6brd1 mutation (rd1-positive) were isolated 5 days after birth and cultivated for 14 days. All micrographs are of the central retina. A, rd1-positive mice lost 90% of photoreceptors, as reflected by the small size of the ONL, which was reduced to a single cell layer. Rd1-positive retinas treated for 12 days with (B) 50 ng/ml of CXCL10 or (C) 100 ng/ml LIF demonstrated rescue of ONL size. D, a combination treatment of 50 ng/ml CXCL10 and 100 ng/ml LIF resulted in slightly increased ONL size relative to the singly treated explants. E, a retinal section of a 20-day-old C57/Bl6 mouse showing the normal size of the ONL in a healthy, non-explanted retina. F, mean ONL thickness for treated and untreated explants. All treatments resulted in significantly increased ONL thicknesses relative to untreated samples (untreated, 9 μm ± 3.9; CXCL10, 21 μm ± 5.5, p < 0.05; LIF, 35 μm ± 7.3, p < 0.05), as well as to the combined treatment (CXCL10/LIF, 38 μm ± 7.9, p < 0.05). None of the treatments restored ONL thickness to the level of C57/Bl6 (wild-type) mice (62 μm ± 11.7). All data presented as mean ± S.D. Scale bar: 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; wt, wild type.
Activation of Pro-survival Signaling through CXCL10 and LIF
Western blot analysis was used to investigate effects of CXCL10 or LIF treatment on intact retinal tissue and isolated PR signal transduction in vitro. We focused on two known survival-associated pathways: signal transducer and activator of transcription (STAT) and B-cell lymphoma (BCL) signaling. Active STAT signaling has been associated with survival in photoreceptors (32) and other cells of the retina (33–35), and BCL-2 is an important anti-apoptotic protein in the retina (36). pSTAT3 levels were markedly increased in explanted porcine retina stimulated with LIF for 24 h, but they were only slightly increased by CXCL10 stimulation (Fig. 5A). Both factors slightly increased BCL-2 levels in explanted retina (Fig. 5A). Stimulation of isolated PRs with LIF also strongly induced pSTAT3 protein levels and only slightly increased BCL-2 levels (Fig. 5B), whereas stimulation with CXCL10 resulted in a stronger induction of BCL-2 levels (Fig. 5B) as compared with pSTAT3 levels.
Fig. 5.
CXCL10 and LIF stimulated anti-apoptotic signaling in the retina. Western blot analyses from porcine explant lysates or primary PR lysates showing levels of pSTAT3 and BCL-2 with α-tubulin as the loading control. pSTAT3 and BCL-2 abundance increased after stimulation with CXCL10 and LIF in the retinal explants (A) and in the isolated porcine PRs (pooled from 20 eyes to ensure adequate material) (B). Signal intensities were determined using ImageJ, normalized against α-tubulin intensities, and are depicted as graphs below the respective Western blot results.
DISCUSSION
RMGs in Health and Disease
The loss of sight is an increasing problem in our progressively aging society, and the search for neurotrophic factors that might alleviate vision loss has been ongoing for the past 40 years. RMGs have been the subject of many investigations, as they have many functions essential to the maintenance of the normal retinal environment, such as metabolite transport (6), control of K+ and water homeostasis (3, 8), and uptake and recycling of neurotransmitters (37). In addition, RMGs release factors that protect neurons, particularly PRs, against cell death (4, 9, 10). Under pathological conditions, RMGs are known to up-regulate glial fibrillary acidic protein (38, 39), exhibit abnormal neurochemical metabolism (40), and have altered expression and function of inwardly rectifying K+ channels (41). Given the importance of RMGs in retinal function, it is important to understand the roles these cells play under normal conditions and how they are altered in disease.
A number of studies from our laboratory have identified neuroprotective factors secreted by RMGs (10, 12). This study continued the search for novel neuroprotective factors by using the quantitative SILAC approach to examine differences in the secretomes of primary porcine RMGs cultivated for either 14 or 21 days. Here, we report the detection SPP1, LIF, transferrin, and CXCL10 in the secretomes of RMGs cultured for 14 days and demonstrate their neuroprotective abilities (Figs. 2 and 4).
Neurotrophic Candidate Proteins
Primary RMGs start losing their neuroprotective capacities when cultivated for over 2 weeks. For this reason, we were interested in proteins that were more abundant on day 14 and assigned to the extracellular compartment. We shortlisted 11 proteins (Table I), of which one candidate, SPP1, had been previously identified and confirmed as a neuroprotective factor via transcriptome analysis (10). In that study, Del Rio et al. induced SPP1 secretion by stimulating RMGs with glial-cell-derived neurotrophic factor, and its neurotrophic activity was demonstrated on primary porcine PRs and Pde6brd1 mouse mutant retinal explants (10). Wahl et al. later demonstrated that RMG-derived SPP1 might exert its neuroprotective effect by preventing cytotoxic RMG swelling (42). The identification of SPP1 in our samples demonstrates the reliability of our proteomic approach. Of the 11 potential factors, we focused on those annotated with “cytokine” function (LIF, CXCL10) and the iron transporter transferrin.
Iron is crucial for cell survival, and stable transferrin expression has been demonstrated to play a major role in iron homeostasis within the retina (43). Iron storage is regulated by ferritin and iron export by proteins such as ceruloplasmin, ferroportin, and hephaestin, and transferrin is involved in iron import into the cell (44). Extracellular transferrin binds iron in circulation to its receptor, which is then internalized through clathrin-coated pits into endosomes (44, 45). Although iron is a co-factor for numerous enzymes and necessary for cell survival, free iron can produce the highly reactive hydroxyl radical, which can damage proteins, lipids, and nucleic acids (16, 43). Iron-induced oxidative stress has been implicated in age-related neurodegenerative diseases affecting the brain and the retina (46). Elevated iron levels have been observed in PRs, the retinal pigment epithelium, and the Bruch's membrane in studies of age-related macular degeneration (16, 45, 46). While examining treatments for increased iron levels in disease, Hadziahmetovic et al. observed beneficial effects when studying the effect of iron chelation on retinal integrity in ferroxidase-deficient mice (47). Picard et al. (16) demonstrated that overexpressed or intraperitoneally injected human transferrin in rd10 mice prevented PR degeneration in vivo, thereby highlighting the important role of transferrin in iron homeostasis in the retina and its neuroprotective capacity. Our observation of greater transferrin abundance in the secretomes of RMGs cultivated for 14 days and demonstration that transferrin promoted PR survival are in accord with these previous studies and might represent a change in RMG metabolism accompanied by the phenotypic de-differentiation toward a fibroblast-like cell. An inability of these de-differentiated cells to bind free iron sufficiently might partially explain their lack of survival-prolonging capacities.
In addition to SPP1 and transferrin, we identified higher levels of LIF in RMG secretomes at day 14 than at day 21. LIF belongs to the IL6 family, also termed glycoprotein 130 cytokines (48). Other members of this cytokine family include ciliary neurotrophic factor, IL11, and oncostatin M, all of which signal through a common glycoprotein 130 receptor (48). LIF exhibits multiple biological effects in the developing vertebrate retina (49) and has been widely investigated as a neurotrophic factor in the retina (14, 50–52). The identification of three previously described neuroprotective molecules validates our quantitative proteomic approach. CXCL10, which has not previously been identified as having neurotrophic properties, was found to have the greatest differential abundance between day 14 and day 21 RMG secretomes, and it was the only cytokine/chemokine other than SPP1 and LIF to demonstrate a differential abundance. CXCL10 is a highly inducible, primary-response protein from the C-X-C chemokine superfamily (53). Pleiotropic biological effects include stimulation of monocytes, natural killer and T-cell migration, regulation of T-cell and bone marrow progenitor maturation, modulation of adhesion molecule expression, and inhibition of angiogenesis (53). CXCL10 and its receptor the chemokine (C-X-C motif) receptor 3 (CXCR3) are expressed throughout the nervous system and have been reported to play a role in acute inflammation (54). Using immunofluorescence, we confirmed that CXCL10 was expressed in healthy porcine retina in vivo (Fig. 3).
Secreted by neurons in response to viral infections, CXCL10 has been discussed as a protective agent that acts by increasing effector T-cell traffic, thereby alleviating viral burden in the brain (55). Experiments on mixed rat hippocampal neuronal and glial cell cultures showed chronically expressed CXCL10 to be protective in certain neuroinflammatory conditions (56). During chronic ocular toxoplasmosis, CXCL10 is required to maintain T-cell populations and to control parasite replication (57). In the retina, CXCL10 has recently been discussed as playing a role in diabetic retinopathy (58).
Roles of CXCL10 and LIF in Neuroprotection
We investigated signal transduction in PRs to elucidate how LIF and CXCL10 mediate neuroprotection. Together with other glycoprotein-130-related cytokines, LIF activates the Janus kinase and STAT pathways (48). The capacity of LIF to counteract oxidative stress (29, 51) fits well with the described role of transferrin in the retina, and as our study found both proteins at reduced levels in de-differentiated cells concomitant with a loss of survival-prolonging capacities, this suggests an important role of RMGs in buffering oxidative stress in the healthy retina. It has been proposed that RMG-derived LIF controls an intrinsic protective mechanism that supports PR cell survival (14). Experiments on RMGs from ciliary neurotrophic factor and LIF knock-out mice demonstrated that the LIF-induced glycoprotein 130/Janus kinase/STAT3 pathway is required for the initiation of the astrogliosis-like reaction of RMGs following optic nerve injury (59). In the context of embryonic stem cells, LIF has recently been shown to induce STAT3 signaling and thereby suppress differentiation. When LIF is withdrawn from the culture medium, the signaling mode of these cells is switched, leading to differentiation (60). If the same signaling mechanism applies to our primary RMGs, reduced LIF production might not only be involved in the loss of neuroprotection but also trigger the de-differentiation process we observed after 21 days in culture.
Western blot analysis demonstrated an increase of pSTAT3 in LIF- and CXL10-stimulated PRs (Fig. 5). This finding agrees with the literature on LIF and, together with our finding that LIF/CXCL10 combined treatment had only a slight additive effect on PR survival in explanted retina, might indicate that CXCL10 and LIF partially act through the same cellular signaling pathway, possibly STAT3 signaling.
So far, little is known regarding the beneficial effect of inflammation in the context of RMG-mediated neuroprotection. While CXCL10 knock-out mice (B6.129S4-Cxcl10tm1Adl/J, The Jackson Laboratory) are viable, albeit with impaired immune functions, no problems in visual function are reported in the literature (61–63). As we were able to demonstrate the neurotrophic potential of CXCL10 in vitro and ex vivo, it can be assumed that CXCL10 acts as an ancillary factor in vivo in concert with other neurotrophic factors. In the context of regeneration, CXCL10 was shown to be hepato-regenerative in a murine model of acute liver injury (64). In retinal ganglion cells, a role for inflammation in the context of regeneration has been discussed, yet the factors involved and the mediating cells remain controversial (65–67).
In addition to STAT signaling, we found BCL-2 to be up-regulated upon CXCL10 stimulation in primary PRs (Fig. 5). Ectopic expression of BCL-2 in PRs of mice with retinal degenerative disease even slowed the progression of disease (68). A recent study by Park et al. reports that changes in retinal glutathione (GSH) levels affect BCL-2 expression in mouse retina (36). GSH plays a critical role in cellular defense against oxidative stress in neurons, and maintenance of GSH levels in the retina is controlled by RMGs (36). The finding that CXCL10-mediated neuroprotection might be BCL-2 associated links an inflammatory molecule to PR survival. One common denominator of neuroprotective factors seems to be the protection against oxidative stress, as demonstrated for transferrin, LIF, and GSH, and future studies will elucidate whether this is also the mechanism for CXCL10. Furthermore, the connection between BCL-2 and GSH expression indicates the loss of neuroprotective capacity of RMGs in cultivation as a lack of detoxification due to the differentiation of RMGs.
CONCLUSION
Primary porcine RMGs are a suitable model for studying RMG-associated neuroprotection. Our quantitative proteomic approach not only validated known neurotrophic factors but also identified a novel candidate in the RMG secretome. Insights into the signaling processes of photoreceptors could help to unravel RMG-mediated neuroprotective signal transduction.
Supplementary Material
Acknowledgments
We thank Juliane Merl, Joanna Kucharska, and Karsten Boldt for constructive discussions and Caroline Bobe and Jennifer Behler for their excellent technical assistance.
Footnotes
Author contributions: C.v.T., M.U., and S.M.H. designed research; C.v., J.M., A.L., and N.S. performed research; C.v. analyzed data; C.v., J.M., A.L., and S.M.H. wrote the paper.
* This work was supported by the German Federal Ministry of Education and Research (HOPE I - FKZ 01GM0852, HOPE II - FKZ 01GM1108A, and SysTec-Verbund IMAGING Grant No. 0315515A) and PRO RETINA Deutschland e. V.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- RMG
- retinal Müller glial cell
- CXCL10
- C-X-C motif chemokine 10
- LIF
- leukemia inhibitory factor
- ONL
- outer nuclear layer
- PR
- photoreceptor
- SILAC
- stable isotope labeling by amino acids in cell culture
- SPP1
- osteopontin
- STAT
- signal transducer and activator of transcription
- pSTAT3
- phosphorylated STAT3
- BCL
- B-cell lymphoma
- GSH
- glutathione
- C-C motif
- Chemokine
- CCL5
- ligand 5
- IL8
- interleukin, C-X-C motif, Chemokine
- receptor 3
- CXCR3.
REFERENCES
- 1. Schnitzer J. (1988) Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia 1, 74–89 [DOI] [PubMed] [Google Scholar]
- 2. Ashwell K. W., Hollander H., Streit W., Stone J. (1989) The appearance and distribution of microglia in the developing retina of the rat. Vis. Neurosci. 2, 437–448 [DOI] [PubMed] [Google Scholar]
- 3. Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N., Reichenbach A. (2006) Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25, 397–424 [DOI] [PubMed] [Google Scholar]
- 4. Reichenbach A., Bringmann A. (2013) New functions of Muller cells. Glia 61, 651–678 [DOI] [PubMed] [Google Scholar]
- 5. Willbold E., Berger J., Reinicke M., Wolburg H. (1997) On the role of Muller glia cells in histogenesis: only retinal spheroids, but not tectal, telencephalic and cerebellar spheroids develop histotypical patterns. J. Hirnforsch 38, 383–396 [PubMed] [Google Scholar]
- 6. Poitry-Yamate C. L., Poitry S., Tsacopoulos M. (1995) Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J. Neurosci. 15, 5179–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnett N. L., Pow D. V. (2000) Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function. Invest. Ophthalmol. Vis. Sci. 41, 585–591 [PubMed] [Google Scholar]
- 8. Winter M., Eberhardt W., Scholz C., Reichenbach A. (2000) Failure of potassium siphoning by Muller cells: a new hypothesis of perfluorocarbon liquid-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 41, 256–261 [PubMed] [Google Scholar]
- 9. Hauck S. M., Kinkl N., Deeg C. A., Swiatek-de Lange M., Schoffmann S., Ueffing M. (2006) GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol. Cell. Biol. 26, 2746–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Rio P., Irmler M., Arango-Gonzalez B., Favor J., Bobe C., Bartsch U., Vecino E., Beckers J., Hauck S. M., Ueffing M. (2011) GDNF-induced osteopontin from Muller glial cells promotes photoreceptor survival in the Pde6brd1 mouse model of retinal degeneration. Glia 59, 821–832 [DOI] [PubMed] [Google Scholar]
- 11. Wenzel A., Grimm C., Samardzija M., Reme C. E. (2005) Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 24, 275–306 [DOI] [PubMed] [Google Scholar]
- 12. Hauck S. M., Gloeckner C. J., Harley M. E., Schoeffmann S., Boldt K., Ekstrom P. A., Ueffing M. (2008) Identification of paracrine neuroprotective candidate proteins by a functional assay-driven proteomics approach. Mol. Cell. Proteomics 7, 1349–1361 [DOI] [PubMed] [Google Scholar]
- 13. Merl J., Ueffing M., Hauck S. M., von Toerne C. (2012) Direct comparison of MS-based label-free and SILAC quantitative proteome profiling strategies in primary retinal Muller cells. Proteomics 12, 1902–1911 [DOI] [PubMed] [Google Scholar]
- 14. Joly S., Lange C., Thiersch M., Samardzija M., Grimm C. (2008) Leukemia inhibitory factor extends the lifespan of injured photoreceptors in vivo. J. Neurosci. 28, 13765–13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Picard E., Fontaine I., Jonet L., Guillou F., Behar-Cohen F., Courtois Y., Jeanny J. C. (2008) The protective role of transferrin in Muller glial cells after iron-induced toxicity. Mol. Vis. 14, 928–941 [PMC free article] [PubMed] [Google Scholar]
- 16. Picard E., Jonet L., Sergeant C., Vesvres M. H., Behar-Cohen F., Courtois Y., Jeanny J. C. (2010) Overexpressed or intraperitoneally injected human transferrin prevents photoreceptor degeneration in rd10 mice. Mol. Vis. 16, 2612–2625 [PMC free article] [PubMed] [Google Scholar]
- 17. Hauck S. M., Dietter J., Kramer R. L., Hofmaier F., Zipplies J. K., Amann B., Feuchtinger A., Deeg C. A., Ueffing M. (2010) Deciphering membrane-associated molecular processes in target tissue of autoimmune uveitis by label-free quantitative mass spectrometry. Mol. Cell. Proteomics 9, 2292–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox J., Matic I., Hilger M., Nagaraj N., Selbach M., Olsen J. V., Mann M. (2009) A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat. Protoc. 4, 698–705 [DOI] [PubMed] [Google Scholar]
- 19. Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V., Mann M. (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 20. Mayburd A. L., Martlinez A., Sackett D., Liu H., Shih J., Tauler J., Avis I., Mulshine J. L. (2006) Ingenuity network-assisted transcription profiling: identification of a new pharmacologic mechanism for MK886. Clin. Cancer Res. 12, 1820–1827 [DOI] [PubMed] [Google Scholar]
- 21. Caffe A. R., Ahuja P., Holmqvist B., Azadi S., Forsell J., Holmqvist I., Soderpalm A. K., van Veen T. (2001) Mouse retina explants after long-term culture in serum free medium. J. Chem. Neuroanat. 22, 263–273 [DOI] [PubMed] [Google Scholar]
- 22. Romijn H. J. (1988) Development and advantages of serum-free, chemically defined nutrient media for culturing of nerve tissue. Biol. Cell 63, 263–268 [DOI] [PubMed] [Google Scholar]
- 23. Ly A., Yee P., Vessey K. A., Phipps J. A., Jobling A. I., Fletcher E. L. (2011) Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest. Ophthalmol. Vis. Sci. 52, 9316–9326 [DOI] [PubMed] [Google Scholar]
- 24. Garcia M., Forster V., Hicks D., Vecino E. (2002) Effects of Muller glia on cell survival and neuritogenesis in adult porcine retina in vitro. Invest. Ophthalmol. Vis. Sci. 43, 3735–3743 [PubMed] [Google Scholar]
- 25. Fuchs C., Forster V., Balse E., Sahel J. A., Picaud S., Tessier L. H. (2005) Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells. Invest. Ophthalmol. Vis. Sci. 46, 2983–2991 [DOI] [PubMed] [Google Scholar]
- 26. Balse E., Tessier L. H., Fuchs C., Forster V., Sahel J. A., Picaud S. (2005) Purification of mammalian cone photoreceptors by lectin panning and the enhancement of their survival in glia-conditioned medium. Invest. Ophthalmol. Vis. Sci. 46, 367–374 [DOI] [PubMed] [Google Scholar]
- 27. Liao L., Park S. K., Xu T., Vanderklish P., Yates J. R., 3rd (2008) Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc. Natl. Acad. Sci. U.S.A. 105, 15281–15286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spellman D. S., Deinhardt K., Darie C. C., Chao M. V., Neubert T. A. (2008) Stable isotopic labeling by amino acids in cultured primary neurons: application to brain-derived neurotrophic factor-dependent phosphotyrosine-associated signaling. Mol. Cell. Proteomics 7, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han Y., Xu J., Li Z., Yang Z. (2013) Neuroprotective effect of leukemia inhibitory factor on antimycin a-induced oxidative injury in differentiated PC12 cells. J. Mol. Neurosci. 50, 577–585 [DOI] [PubMed] [Google Scholar]
- 30. Arango-Gonzalez B., Cellerino A., Kohler K. (2009) Exogenous brain-derived neurotrophic factor (BDNF) reverts phenotypic changes in the retinas of transgenic mice lacking the BDNF gene. Invest. Ophthalmol. Vis. Sci. 50, 1416–1422 [DOI] [PubMed] [Google Scholar]
- 31. Farber D. B., Flannery J. G., Bowes-Rickman C. (1994) The rd mouse story: seventy years of research on an animal model of inherited retinal degeneration. Prog. Ret. Eye Res. 13, 31–64 [Google Scholar]
- 32. Chucair-Elliott A. J., Elliott M. H., Wang J., Moiseyev G. P., Ma J. X., Politi L. E., Rotstein N. P., Akira S., Uematsu S., Ash J. D. (2012) Leukemia inhibitory factor coordinates the down-regulation of the visual cycle in the retina and retinal-pigmented epithelium. J. Biol. Chem. 287, 24092–24102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel A. K., Hackam A. S. (2013) Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol. Immunol. 54, 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin P. K., Ke C. Y., Khor C. N., Cai Y. J., Lee Y. J. (2013) Involvement of SDF1a and STAT3 in granulocyte colony-stimulating factor rescues optic ischemia-induced retinal function loss by mobilizing hematopoietic stem cells. Invest. Ophthalmol. Vis. Sci. 54, 1920–1930 [DOI] [PubMed] [Google Scholar]
- 35. Li R., Wen R., Banzon T., Maminishkis A., Miller S. S. (2011) CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One 6, e23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park M. H., Kim S. Y., Moon C., Bae Y. C., Moon J. I. (2013) Differential cell death and Bcl-2 expression in the mouse retina after glutathione decrease by systemic D,L-buthionine sulphoximine administration. Mol. Cells 35, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bringmann A., Grosche A., Pannicke T., Reichenbach A. (2013) GABA and glutamate uptake and metabolism in retinal glial (Muller) cells. Front. Endocrinol. 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang L., Cioffi G. A., Cull G., Dong J., Fortune B. (2002) Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest. Ophthalmol. Vis. Sci. 43, 1088–1094 [PubMed] [Google Scholar]
- 39. Mizutani M., Gerhardinger C., Lorenzi M. (1998) Muller cell changes in human diabetic retinopathy. Diabetes 47, 445–449 [DOI] [PubMed] [Google Scholar]
- 40. Ishikawa A., Ishiguro S., Tamai M. (1996) Changes in GABA metabolism in streptozotocin-induced diabetic rat retinas. Curr. Eye Res. 15, 63–71 [DOI] [PubMed] [Google Scholar]
- 41. Iandiev I., Uckermann O., Pannicke T., Wurm A., Tenckhoff S., Pietsch U. C., Reichenbach A., Wiedemann P., Bringmann A., Uhlmann S. (2006) Glial cell reactivity in a porcine model of retinal detachment. Invest. Ophthalmol. Vis. Sci. 47, 2161–2171 [DOI] [PubMed] [Google Scholar]
- 42. Wahl V., Vogler S., Grosche A., Pannicke T., Ueffing M., Wiedemann P., Reichenbach A., Hauck S. M., Bringmann A. (2013) Osteopontin inhibits osmotic swelling of retinal glial (Muller) cells by inducing release of VEGF. Neuroscience 246, 59–72 [DOI] [PubMed] [Google Scholar]
- 43. Loh A., Hadziahmetovic M., Dunaief J. L. (2009) Iron homeostasis and eye disease. Biochim. Biophys. Acta 1790, 637–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rouault T. A. (2013) Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 551–564 [DOI] [PubMed] [Google Scholar]
- 45. He X., Hahn P., Iacovelli J., Wong R., King C., Bhisitkul R., Massaro-Giordano M., Dunaief J. L. (2007) Iron homeostasis and toxicity in retinal degeneration. Prog. Retin. Eye Res. 26, 649–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hadziahmetovic M., Song Y., Ponnuru P., Iacovelli J., Hunter A., Haddad N., Beard J., Connor J. R., Vaulont S., Dunaief J. L. (2012) Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Invest. Ophthalmol. Vis. Sci. 52, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hadziahmetovic M., Song Y., Wolkow N., Iacovelli J., Grieco S., Lee J., Lyubarsky A., Pratico D., Connelly J., Spino M., Harris Z. L., Dunaief J. L. (2011) The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration. Invest. Ophthalmol. Vis. Sci. 52, 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zigmond R. E. (2011) gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front. Mol. Neurosci. 4, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rhee K. D., Yang X. J. (2010) Function and mechanism of CNTF/LIF signaling in retinogenesis. Adv. Exp. Med. Biol. 664, 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ueki Y., Wang J., Chollangi S., Ash J. D. (2008) STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J. Neurochem. 105, 784–796 [DOI] [PubMed] [Google Scholar]
- 51. Chollangi S., Wang J., Martin A., Quinn J., Ash J. D. (2009) Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol. Dis. 34, 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burgi S., Samardzija M., Grimm C. (2009) Endogenous leukemia inhibitory factor protects photoreceptor cells against light-induced degeneration. Mol. Vis. 15, 1631–1637 [PMC free article] [PubMed] [Google Scholar]
- 53. Neville L. F., Mathiak G., Bagasra O. (1997) The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 8, 207–219 [DOI] [PubMed] [Google Scholar]
- 54. Klein R. S. (2004) Regulation of neuroinflammation: the role of CXCL10 in lymphocyte infiltration during autoimmune encephalomyelitis. J. Cell. Biochem. 92, 213–222 [DOI] [PubMed] [Google Scholar]
- 55. Klein R. S., Lin E., Zhang B., Luster A. D., Tollett J., Samuel M. A., Engle M., Diamond M. S. (2005) Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79, 11457–11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bajova H., Nelson T. E., Gruol D. L. (2008) Chronic CXCL10 alters the level of activated ERK1/2 and transcriptional factors CREB and NF-kappaB in hippocampal neuronal cell culture. J. Neuroimmunol. 195, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Norose K., Kikumura A., Luster A. D., Hunter C. A., Harris T. H. (2011) CXCL10 is required to maintain T-cell populations and to control parasite replication during chronic ocular toxoplasmosis. Invest. Ophthalmol. Vis. Sci. 52, 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nawaz M. I., Van Raemdonck K., Mohammad G., Kangave D., Van Damme J., Abu El-Asrar A. M., Struyf S. (2013) Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp. Eye Res. 109, 67–76 [DOI] [PubMed] [Google Scholar]
- 59. Kirsch M., Trautmann N., Ernst M., Hofmann H. D. (2010) Involvement of gp130-associated cytokine signaling in Muller cell activation following optic nerve lesion. Glia 58, 768–779 [DOI] [PubMed] [Google Scholar]
- 60. Liu J. W., Hsu Y. C., Kao C. Y., Su H. L., Chiu I. M. (2013) Leukemia inhibitory factor-induced Stat3 signaling suppresses fibroblast growth factor 1-induced Erk1/2 activation to inhibit the downstream differentiation in mouse embryonic stem cells. Stem Cells Dev. 22, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 61. Hamrah P., Yamagami S., Liu Y., Zhang Q., Vora S. S., Lu B., Gerard C. J., Dana M. R. (2007) Deletion of the chemokine receptor CCR1 prolongs corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 48, 1228–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dufour J. H., Dziejman M., Liu M. T., Leung J. H., Lane T. E., Luster A. D. (2002) IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168, 3195–3204 [DOI] [PubMed] [Google Scholar]
- 63. Christensen J. E., de Lemos C., Moos T., Christensen J. P., Thomsen A. R. (2006) CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J. Immunol. 176, 4235–4243 [DOI] [PubMed] [Google Scholar]
- 64. Bone-Larson C. L., Hogaboam C. M., Evanhoff H., Strieter R. M., Kunkel S. L. (2001) IFN-gamma-inducible protein-10 (CXCL10) is hepatoprotective during acute liver injury through the induction of CXCR2 on hepatocytes. J. Immunol. 167, 7077–7083 [DOI] [PubMed] [Google Scholar]
- 65. Leon S., Yin Y., Nguyen J., Irwin N., Benowitz L. I. (2000) Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 20, 4615–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yin Y., Cui Q., Li Y., Irwin N., Fischer D., Harvey A. R., Benowitz L. I. (2003) Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 23, 2284–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leibinger M., Muller A., Andreadaki A., Hauk T. G., Kirsch M., Fischer D. (2009) Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 29, 14334–14341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eversole-Cire P., Concepcion F. A., Simon M. I., Takayama S., Reed J. C., Chen J. (2000) Synergistic effect of Bcl-2 and BAG-1 on the prevention of photoreceptor cell death. Invest. Ophthalmol. Vis. Sci. 41, 1953–1961 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.