Abstract
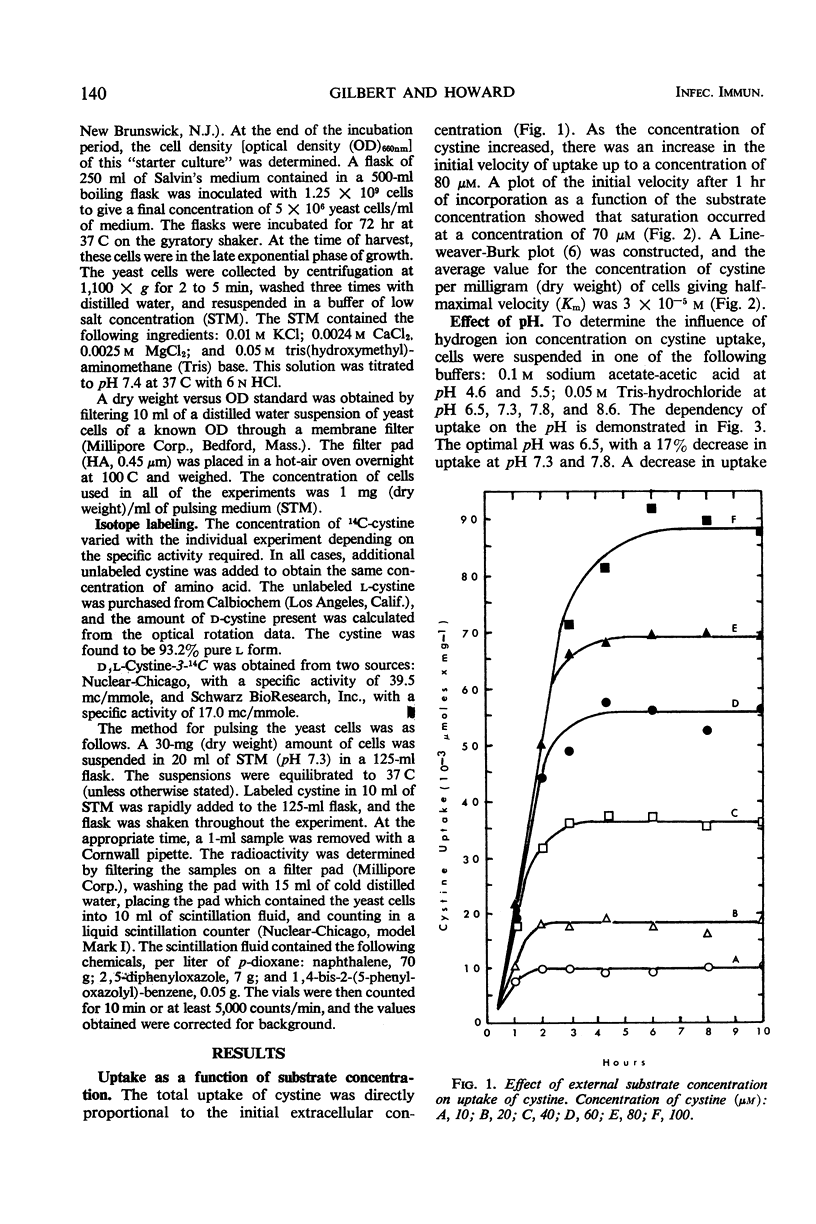
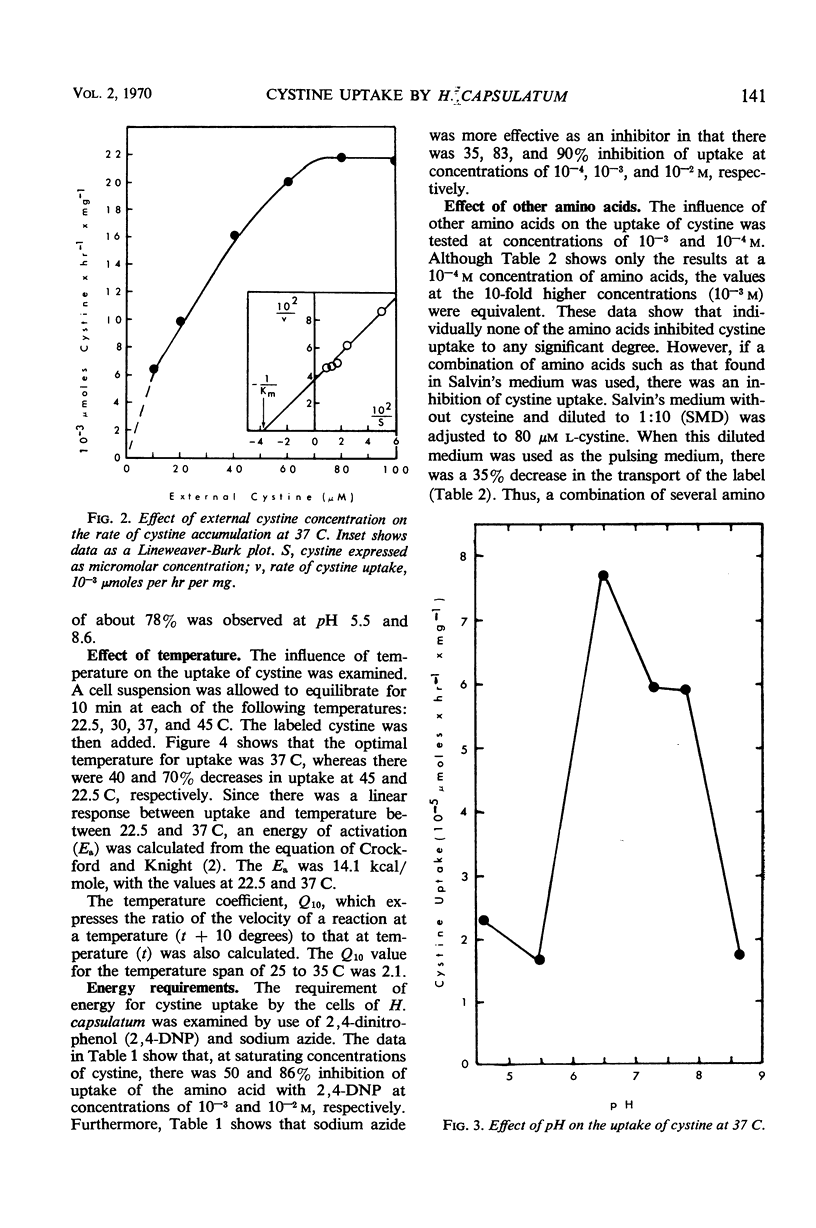
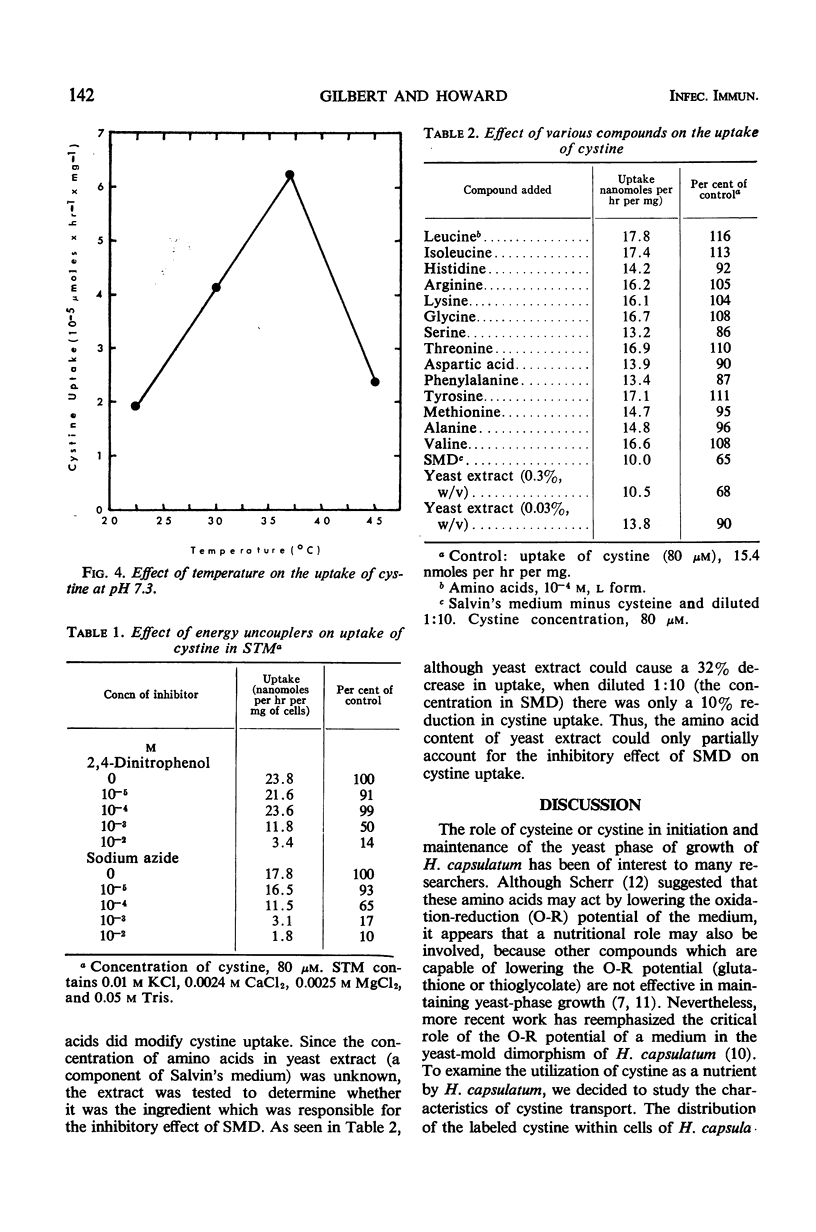
This report deals with factors affecting the uptake of cystine by the yeast phase of Histoplasma capsulatum. The kinetics of uptake showed a saturation at 70 μM and an average Km value of 3 × 10−5m. The optimal pH and temperature for transport of cystine were 6.5 and 37 C, respectively. The energy of activation was 14.1 kcal/mole, and the temperature coefficient value was 2.1. A requirement for energy supplied by metabolic activity was demonstrated by the inhibition of incorporation of the amino acid by cells preincubated with either 2,4-dinitrophenol or sodium azide. Although uptake was not inhibited by any single amino acid, a combination of amino acids did cause a decrease in uptake. Thus, the data show that the uptake of cystine by yeast cells of H. capsulatum has the characteristics of a system of transport that requires the expenditure of energy by the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeBusk B. G., DeBusk A. G. Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta. 1965 Jun 15;104(1):139–150. doi: 10.1016/0304-4165(65)90229-1. [DOI] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Pramer D. Amino acid transport by the filamentous fungus Arthrobotrys conoides. J Bacteriol. 1970 Jul;103(1):120–130. doi: 10.1128/jb.103.1.120-130.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh I., Morton K. Nutritional studies of Histoplasma capsulatum. Mycopathol Mycol Appl. 1965 Apr 14;25(3):294–308. doi: 10.1007/BF02049917. [DOI] [PubMed] [Google Scholar]
- PINE L. Studies on the growth of Histoplasma capsulatum. I. Growth of the yeast phase in liquid media. J Bacteriol. 1954 Dec;68(6):671–679. doi: 10.1128/jb.68.6.671-679.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Gottfried L., Rottem S. Amino acid transport in Mycoplasma. J Bacteriol. 1968 May;95(5):1685–1691. doi: 10.1128/jb.95.5.1685-1691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon J. W. Monitored environment system to control cell growth, morphology, and metabolic rate in fungi by oxidation-reduction potentials. Appl Microbiol. 1968 Jan;16(1):114–121. doi: 10.1128/am.16.1.114-121.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERR G. H. Studies on the dimorphism of Histoplasma capsulatum. I. The roles of -SH groups and incubation temperature. Exp Cell Res. 1957 Feb;12(1):92–107. doi: 10.1016/0014-4827(57)90296-3. [DOI] [PubMed] [Google Scholar]
- SOLOMON A. K. The permeability of the human erythrocyte to sodium and potassium. J Gen Physiol. 1952 May;36(1):57–110. doi: 10.1085/jgp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. I. Specificity and kinetics. J Bacteriol. 1966 Dec;92(6):1698–1705. doi: 10.1128/jb.92.6.1698-1705.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


