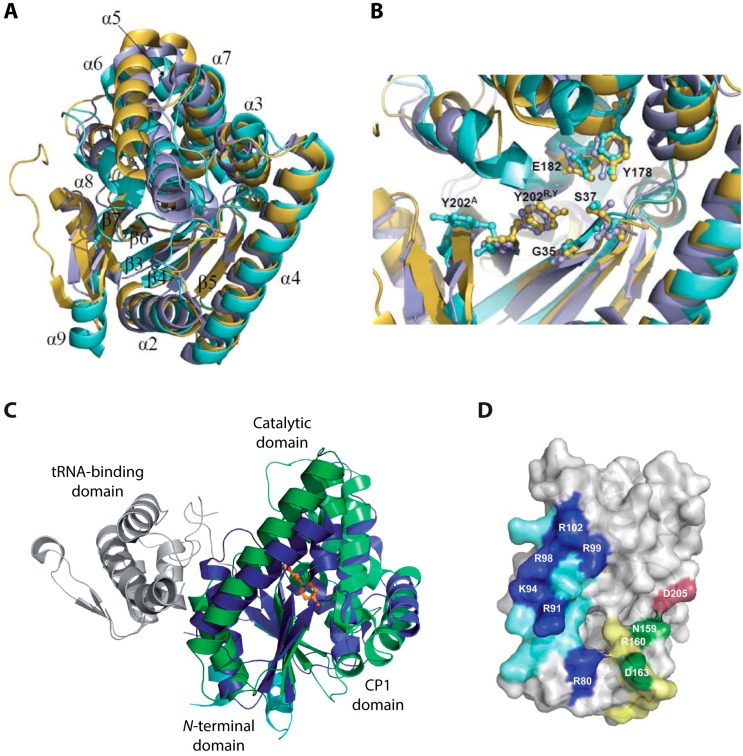
Figure 5.
(A) Superposition of the AlbC (cyan, PDB (Protein Data Bank) ID: 3OQV), Rv2275 (yellow, PDB ID: 2X9Q) and YvmC-Blic (purple, PDB ID: 3OQH) structures. The numbering of β-strands and α-helices corresponds to AlbC; (B) Superposition of the conserved residues inside the active site pocket. The residues are shown in ball and stick style. AlbC numbering is used; (C) Comparison of the structures of AlbC and TyrRSTyr from M. jannaschii in complex with l-Tyr (PDB ID: 1JLU). The enzymes are shown in cartoon style, l-Tyr is shown in ball and stick style in orange. The Rossmann-fold and CP1 domain of TyrRSTyr are colored in dark and light blue, respectively. The corresponding domains of AlbC are shown in dark and light green. The tRNA-binding domain is colored grey; and (D) Regions in AlbC involved in the interaction with tRNA substrates (PDB ID: 3OQV). Basic residues located in helix α4 are colored in blue. Residues shown in dark blue have been shown to strongly influence the interaction with the first aminoacyl-tRNA substrate. Loop α6-α7 is colored in yellow and D205 in loop β6-α8 in red. Green and red residues have been shown to influence binding of the second tRNA substrate. (A,B) Adapted from [31] with permission of The Royal Society of Chemistry, copyright 2012; (C) Adapted from [44] with permission from Oxford University Press, copyright 2011; and (D) Adapted from [52] with permission from Oxford University Press, copyright 2014.