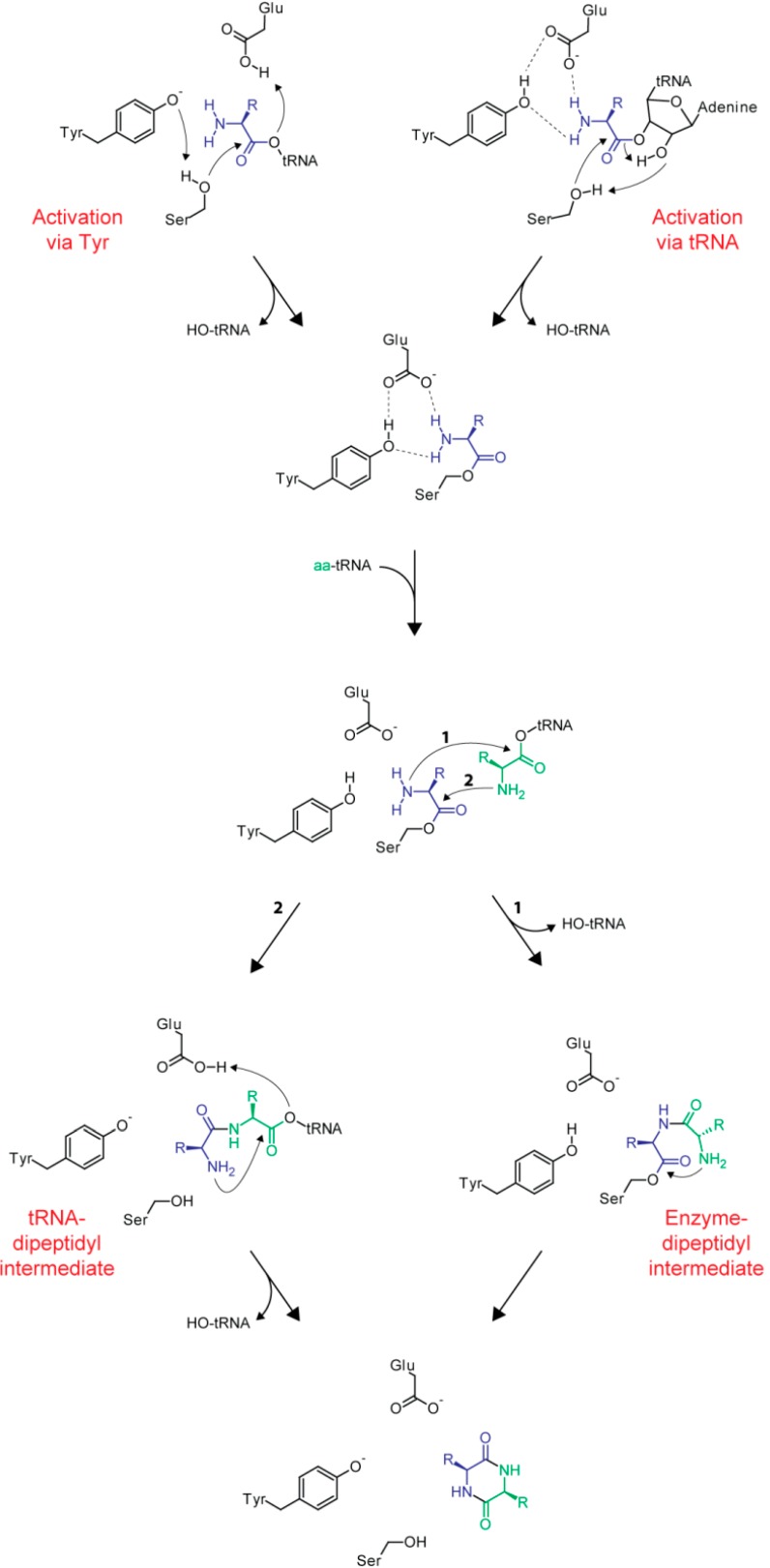
Figure 6.
Proposed ping-pong mechanism of CDPS catalysis. Activation of the active site nucleophile (Ser) can be accomplished through interaction with a conserved tyrosine (left) or via a proton-shuttling mechanism involving the tRNA substrate (right). After the covalently bound aminoacyl-enzyme intermediate has been formed either an enzyme-dipeptidyl (right) or tRNA-dipeptidyl (left) intermediate is generated. Through attack of the α-amino group of the second aminoacyl moiety, the CDP is formed and released from the enzyme.