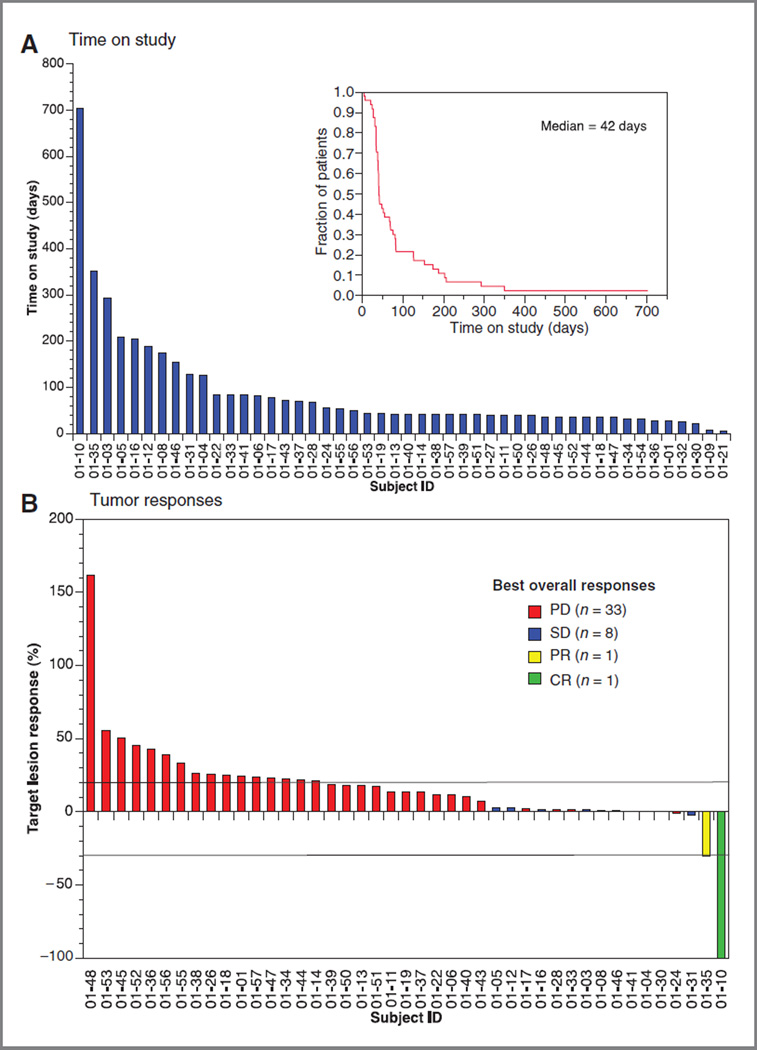
Figure 1.
A, the median time on study was 6 weeks (95% CI, 1–101+ weeks). Forty-three (89.5%) patients came off study because of progression of disease, 2 patients because of toxicity, and 1 patient died. One patient withdrew consent. One patient remained on study at the time of analysis. B, waterfall plot of tumor responses of the 43 patients for whom serial imaging studies were available. One patient achieved a CR, 1 patient a prolonged PR, and 8 patients achieved stable disease for12 or more weeks (range, 12–36 weeks).