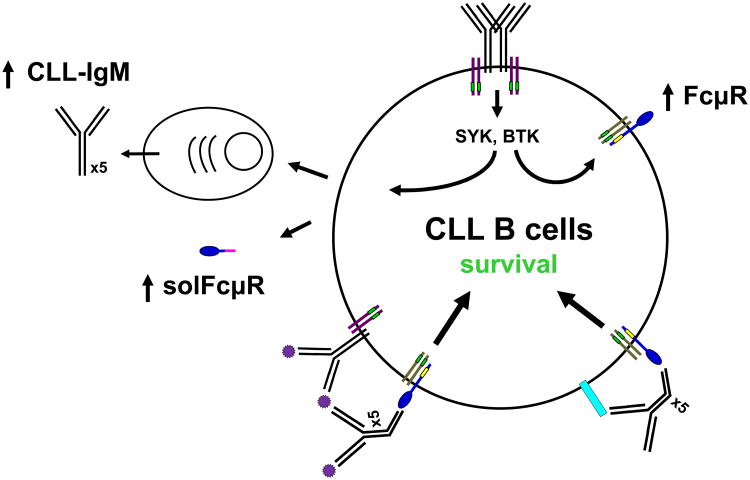
Figure 8.
Hypothetical role of Fc μR in CLL. Our current working hypothesis of the role of FcμR in CLL is as follows. Antigen-independent self-ligation of BCR on CLL cells activates SYK and BTK tyrosine kinases and induces up-regulation of the cell surface expression of FcμR/adaptor protein complex. Subpopulations of CLL cells differentiate into plasma cells that secrete pentameric IgM antibodies. Secreted IgM antibodies recognize soluble (purple spiky small circles) or lymphocyte membrane (light blue rectangle) self-antigens. The colligation of FcμR and BCR by soluble IgM/self-antigen immune complexes or the cis interaction of FcμR and lymphocyte membrane self-antigen by secreted IgM provides a survival signal to CLL cells through FcμR. BCR is depicted as black Y shape heavy and light chain lines with Igα/β adaptor proteins (purple lines) carrying ITAM (small green rectangles). FcμR ligand-binding chain is depicted as a blue tennis racket shape with a small yellow rectangle indicating three conserved intracytoplasmic Tyr residues and associates with an unknown adaptor protein (gray lines) possibly carrying ITAM (small green rectangles). Soluble FcμR, an alternative splice variant, is also markedly elevated in CLL patients, but its biological function remains unknown.