Figure 6.
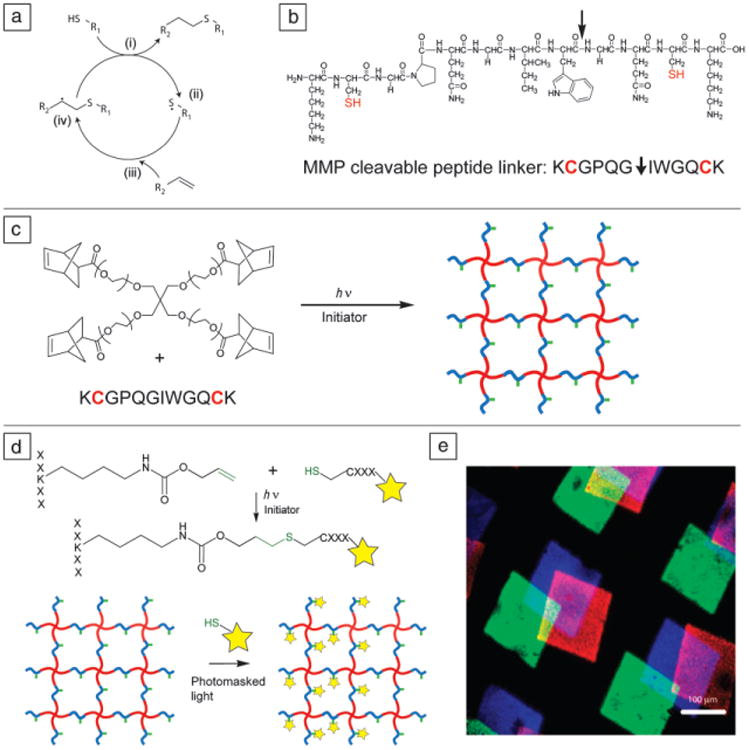
(a) Thiol-ene reaction cycle. An initiator abstracts a proton from a thiol (i), forming a thiyl radical (ii), which then reacts with a carbon-carbon double bond (iii). The resulting carbon radical (iv) abstracts a proton from another thiol, completing the thio-ether bond and regenerating a thiyl radical. (b) Di-cysteine polypeptide monomer, chemical structure (top) and amino acid abbreviation (bottom). This sequence, derived from collagen I, is cleavable by cell-secreted matrix metalloproteinases (MMPs) (cleavage site indicated by arrow) and is often used as a cellularly degradable cross-linker. (c) PEG-tetranorbornene and di-cysteine peptides react via the thiol-ene reaction cycle to form a step-growth network. Note: PEG (red), peptide cross-linker (blue), pendant peptide (green). (d) Peptide tethering with the thiol-ene reaction. Using photomasked light or focused laser light, cysteine-containing peptides can be covalently attached to pendant ene groups on the polymer network exclusively in user-defined regions. Fluorescent label on pendant peptide is indicated by a star. (e) Three different fluorescently labeled peptides (blue, green, and red) are sequentially attached at user-defined locations and times using photomasks and radical-initiated thiol-ene coupling reactions. Adapted from References 50, 54, and 55. Note: PEG, polyethylene glycol); h, Planck constant; ν, frequency of light; R1 and R2, side chains; X, any amino acid residue.
