Figure 8.
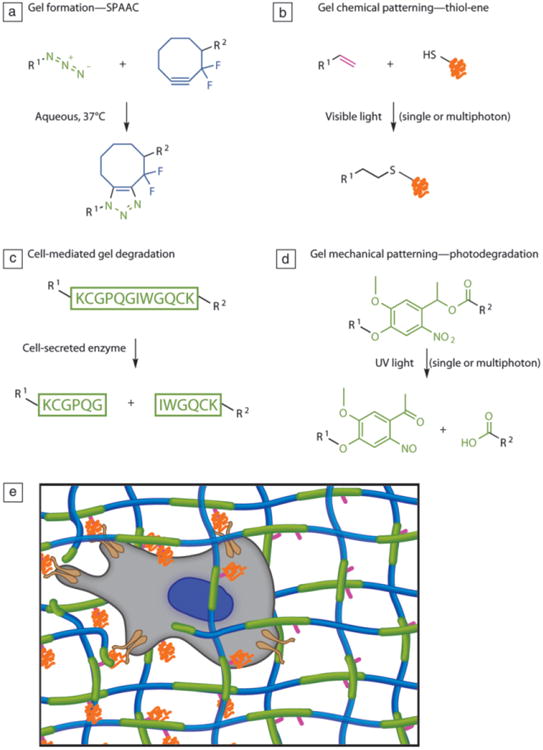
Sequential and orthogonal reactions. (a) Gels can be formed via a strain-promoted azide-alkyne cycloaddition (SPAAC) between cyclooctyne-functionalized polyethylene glycols) (PEGs, blue) and azide-functionalized peptides (green). (b) Peptide ligands can be patterned into the gel through the photoinitiated thiol-ene reaction between a cysteine-containing peptide (orange) and a free carbon-carbon double bond (pink) from the alloc protecting group located on the peptide cross-linker. (c) By choosing an enzymatically cleavable peptide sequence for the di-azide cross-linker (green), the gel can be locally degraded by cells. (d) User-dictated degradation can be accomplished by incorporating a photolabile moiety (green) within the cross-linker. (e) In this synthetic scaffold, the cell (gray) can attach to the PEG matrix (blue) through integrins (brown) binding to adhesive peptides (orange), which are covalently linked to the network through a pendant ene functionality (pink) during a post-gelation photoinitiated reaction, enabling spatial patterning of the peptide. Enzymatically degradable peptide sequences and/or photolabile groups form the cross-links (green), allowing for cellular and/or user remodeling of the microenvironment. Adapted from Reference 56. Note: R1 and R2, side chains.
