Scheme 1. Synthesis of (−)-Methyl Atisenoate (3) and (−)-Isoatisine (4).
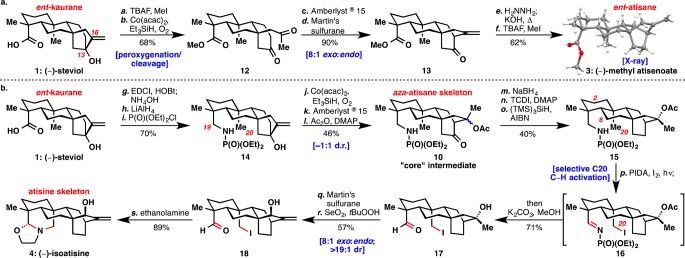
Reagents and conditions: (a) Methyl iodide (1.2 equiv), TBAF (1.2 equiv), THF, 23 °C, 16 h (91%); (b) TESH (2.2 equiv), Co(acac)2 (0.2 equiv), O2 (balloon), DCE, 40 °C, 6 h (75%); (c) Amberlyst 15 resin (0.5 mg/mg substrate), acetone, 40 °C, 3 h (98%); (d) Martin’s sulfurane (2 equiv), CH2Cl2, −78 °C to room temp. (91%); (e) H2NNH2 (20 equiv), diethylene glycol, 100 °C, 90 min; KOH (5 equiv), 200 °C, 21 h (73%); (f) MeI (1.5 equiv), TBAF (1.5 equiv), THF, 23 °C, 3 h (85%); (g) HOBt·H2O (1.4 equiv), EDCI·HCl (2.8 equiv), NH4OH, THF, 23 °C, 36 h (80%); (h) LiAlH4 (5 equiv), THF, 70 °C, 48 h, (94%); (i) (iPr)2NEt (6 equiv), P(O)(OEt)2Cl (3 equiv), ACN, 60 °C, 16 h (93%); (j) TESH (2.2 equiv), Co(acac)2 (0.2 equiv), O2 (balloon), DCE, 40 °C, 16 h (59%); (k) Amberlyst 15 resin (0.5 mg/mg substrate), acetone, 40 °C, 3 h (87%); (l) Ac2O (5 equiv), DMAP (1 equiv), CHCl3/Et3N (1:1), 40 °C, 16 h (90%); (m) NaBH4 (3 equiv), MeOH, 0 to 23 °C; (n) TCDI (4 equiv), DCE, 80 °C, 18 h; (o) (TMS)3SiH (10 equiv), AIBN (0.5 equiv), dioxane, 80 °C (40% over 3 steps); (p) PIDA (4 equiv), I2 (5 equiv), DCE, 90-W sunlamp, 40 °C, 40 min; K2CO3 (25 equiv), MeOH, 65 °C, 36 h (71%); (q) Martin’s sulfurane (2 equiv), CH2Cl2, −78 to 23 °C; (r) SeO2 (4 equiv), tBuOOH (30 equiv), CH2Cl2, 0 °C, 1 h (63%, 2 steps), (s) ethanolamine (3 equiv), MeOH, 23 °C (89%). THF = tetrahydrofuran, TBAF = tetra-n-butyl ammonium fluoride, TESH = triethylsilane, DCE = 1,2-dichloroethane, HOBt = hydroxybenzotriazole, EDCI = 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide, ACN = acetonitrile, DMAP = 4-dimethylaminopyridine, TCDI = 1,1′-thiocarbonyldiimidazole, AIBN = azobis(isobutyronitrile), PIDA = phenyliodine diacetate.
