Table 2.
Effects of the catalyst on the fluorination of 5[a]
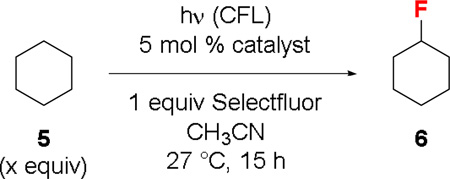 | ||||
|---|---|---|---|---|
| Entry | 5 (equiv) | ketone catalyst | Yield[b] | |
| 1 | 23 | 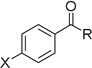 |
R = CH3, X = H | 90% |
| 2 | 23 | R = CH3, X = OMe | 82% | |
| 3 | 23 | R = CH3, X = NO2 | 18% | |
| 4 | 23 | R = CF3, X = H | 63% | |
| 5 | 23 | R = H, X = H | 59% | |
| 6 | 23 | 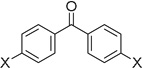 |
X = H | 78% |
| 7 | 23 | X = OMe | 76% | |
| 8 | 23 | X = NMe2 | 17% | |
| 9 | 23 | 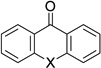 |
X = – | 75% |
| 10 | 23 | X = O | 87% | |
| 11 | 5 | X = O | 81% | |
| 12 | 5 | 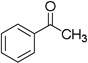 |
88% | |
| 13 | 1.5 | 76% | ||
| 14 | 1.0 | 59% | ||
Reaction conditions: 0.01 mmol catalyst, 0.2 mmol Selectfluor, 2 mL CH3CN.
Determined by 19F NMR using C6H5F as an external standard.
