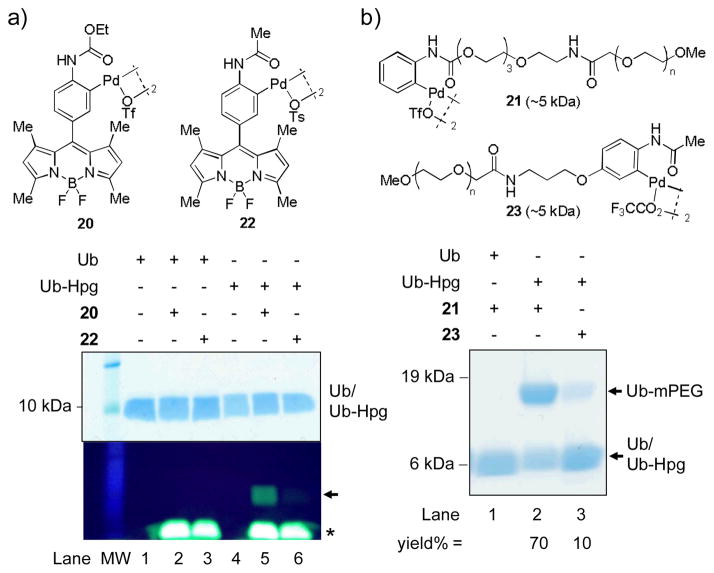
Figure 2.
Reactivity comparison of the N-phenylcarbamate palladacycles vs. the acetanilide palladacycles in the bioconjugation reaction with Ub-Hpg. (a) Coomassie blue stained SDS-PAGE (top) and in-gel fluorescence analysis (bottom) of the product mixture after incubating 2.5 μM Ub-Hpg with 10 μM palladacycle 20 or 22 in PBS for 1 min:λex = 365 nm. The fluorescent labeled product band is indicated with an arrow. The front migrating excess palladacycles are indicated with an asterisk. 15% SDS-PAGE gel was used. (b) Coomassie blue stained SDS-PAGE gel of the PEGylation product mixtures after incubating 2.5 μM Ub-Hpg with 10 μM palladacycle 21 or 23 in PBS for 1 min. The PEGylated product band is indicated with an arrow. Bio-Rad Any kD Mini-PROTEAN TGX Gel was used.