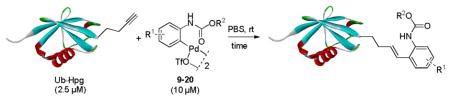
Table 2.
Reactivity of Substituted N-Phenylcarbamate Palladacycles toward Ub- Hpga
| |||
|---|---|---|---|
| entry | palladacycles | conversion (%)b
|
|
| 10 s | 3 min | ||
| 1 |
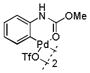 9 |
83 | >99 |
| 2 |
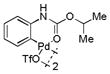 10 |
71 | >99 |
| 3 |
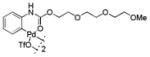 11 |
79 | 97 |
| 4 |
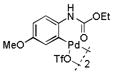 12 |
42 | >99 |
| 5 |
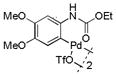 13 |
40 | >99 |
| 6 |
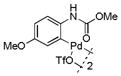 14 |
49 | >99 |
| 7 |
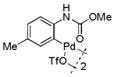 15 |
74 | >99 |
| 8 |
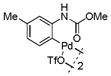 16 |
80 | >99 |
| 9 |
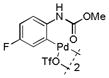 17 |
74 | >99 |
| 10 |
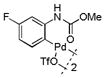 18 |
35 | >99 |
| 11 |
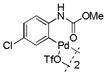 19 |
41 | >99 |
| 12 |
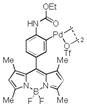 20 |
41 | 53 |
Reaction was carried out by incubating 2.5 μM Ub-Hpg with 10 μM palladacycle at room temperature for the indicated time (see detail procedure in SI, general procedures for the reaction of palladacycles with Ub-Hpg).
Conversion was determined based on LC-MS analysis: conversion % = Iproduct/(IUb-Hpg + Iproduct), where IUb-Hpg and Iproduct represent the ion counts of Ub-Hpg and the product, respectively.
