Abstract
Background
The Bernese periacetabular osteotomy (PAO) traditionally is performed using the iliofemoral or the ilioinguinal approach with transection of the rectus femoris tendon attachments. Although a rectus-preserving approach has been developed, there is limited direct comparison data regarding the surgical safety, radiographic correction, and improvement in hip pain and function between the rectus-preserving and the classic approaches.
Questions/purposes
The purposes of this study were to determine whether preserving the rectus femoris tendon attachment would (1) reduce intraoperative blood loss and length of surgery; (2) improve Harris hip scores (HHS); (3) decrease the rate of complications; and (4) affect the radiographic correction when compared with the classic approach.
Methods
A retrospective matched cohort study was used to compare the endpoints listed above after PAO using a rectus-preserving approach versus the classic approach. Operative blood loss, preoperative and postoperative hematocrit, duration of surgery, HHS, and postoperative complications were recorded for the two groups. Pelvic radiographs were reviewed for measurement of the lateral center-edge angle, anterior center-edge angle, and Tönnis acetabular inclination angle. A total of 64 patients were included (32 in each group). Followup was at a minimum of 1 year (mean, 20 months; range, 13–44 months).
Results
Blood loss (p = 0.2405), hematocrit change (p = 0.3277), and operative time (p = 0.3960) were similar between groups. At latest followup, the HHS improved in the rectus-preserving (mean improvement, 25; 95% CI, 21–29; p < 0.0001) and control groups (mean improvement, 21; 95% CI, 17–25; p < 0.0001) with no difference in HHS improvement between the groups (mean difference, 4.3; 95% CI, −1.6 to 10.1; p = 0.1523). The complication rate was 12.5% (four of 32) in the rectus-preserving group and 25% (eight of 32) in the classic approach groups, respectively (p = 0.2002). The rectus-preserving approach allowed for similar lateral center-edge angle (p = 0.4463), anterior center-edge angle (p = 0.0936), and Tönnis angle (p = 0.7953) improvement when compared with the classic approach.
Conclusions
The rectus-preserving approach for PAO is as safe and effective as the classic approach to achieve radiographic correction and HHS improvement at minimum 1 year. Additional investigation is needed to determine whether the rectus-preserving approach allows for improvement in functional recovery including hip flexion strength.
Level of Evidence
Level III, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
The Bernese periacetabular osteotomy (PAO) is performed for treatment of symptomatic acetabular dysplasia in the young adult [6, 11, 15, 16, 20, 25, 27]. The original technique described by Ganz et al. [6] used an iliofemoral exposure (modified Smith-Petersen [19]) involving extensive abductor dissection and transection of the indirect and direct heads of the rectus femoris tendon. Since the original description, there have been several reports describing different surgical approaches to perform a PAO. These include the ilioinguinal approach [8, 17, 23, 27], a direct anterior approach without abductor dissection [13], and a “minimally invasive transsartorial approach” [24]. However, there are few studies comparing clinical and functional outcomes between these different approaches [8, 23].
The surgical approach used to perform a PAO may influence the duration of the procedure, the volume of blood loss during surgery, and affect the accuracy of radiographic correction of the acetabulum and the occurrence of complications. For several years, the senior authors (MBM, YJK) performed PAO using the modified anterior approach [13] on a routine basis. Increasing interest in reducing the morbidity and soft tissue damage led us to start preserving the indirect and direct head of the rectus femoris tendon in selected cases. However, to our knowledge, this approach has not been compared with the classic approach.
In this study we sought to determine whether preserving the rectus femoris tendon attachment would (1) reduce intraoperative blood loss and length of surgery (2) improve the Harris hip scores (HHS) at minimum 1 year; (3) decrease the rate of complications; and (4) affect the radiographic correction when compared with the classic approach.
Patients and Methods
After approval of our institutional review board, a query of the orthopaedic surgical database at our institution was performed to identify patients who underwent a Bernese PAO for treatment of symptomatic hip dysplasia between October 2009 and June 2011. During this period, a total of 32 patients underwent PAO using the rectus-preserving technique. General indications for the rectus-preserving technique include: acetabular dysplasia secondary to developmental dysplasia of the hip (DDH), negative history of previous surgery, no history of locking or catching of the hip, no major femoral head deformities observed on radiographs, no full-thickness labral tear detected on MR images, and minimum of 100° hip flexion and 20° internal rotation with the hip flexed at 90°. All 32 patients were followed for a minimum of 12 months. During that time, we also performed 141 procedures using the classic technique, because these patients did not meet our general indications for the rectus-preserving technique.
The patients who had a rectus-preserving PAO then were matched to patients who underwent PAO through a classic approach (control group). To increase the chance of finding a match for every patient in the rectus-preserving group we searched the database for patients who underwent a PAO from May 2006 to June 2011. A total of 435 patients underwent a PAO during this 5-year period (32 rectus-preserving and 403 classic approach). For the matching process only patients with acetabular dysplasia secondary to DDH were included. Patients with a primary or concurrent diagnosis of a hip disorder other than DDH, patients with major femoral head-neck deformity related to Legg-Calvé-Perthes disease or other, patients with less than 1 year clinical followup, and patients with previous hip surgery were excluded from the match process. A modified 1:1 nearest neighbor matching approach was used to match subjects in the control cohort to subjects in the rectus cohort on the basis of diagnosis of acetabular dysplasia secondary to DDH, sex, age at surgery, and BMI [3]. Accordingly, subjects in the control cohort were stratified in blocks based on primary diagnosis, sex, and age. Subjects in the rectus-preserving group were assigned to an appropriate block and subsequently, in these blocks; the subjects in the two groups that were closest in BMI were matched together.
All patients presented with a history of hip pain for at least 3 months, radiographic criteria of hip dysplasia (lateral center-edge angle of Wiberg [28] 20° or less or anterior center-edge angle [10] 20° or less, or both), and radiographic evidence of minimal or no hip osteoarthritis (Tönnis [22] Grade 0 or 1). No patients were contacted specifically for this study and the data from all patients were obtained from retrospective assessment of medical records, and previously, prospectively collected hip-specific questionnaires obtained during the clinical visit. A total of 64 patients were included (32 in each group). Followup was at a minimum of 1 year (mean, 20 months; range, 13–44 months). There were no differences (p > 0.05) between groups with respect to sex, age at surgery, BMI, operative side, or length of followup (Table 1). For each patient, sex, age at the time of surgery, BMI, operative findings, complications, and subsequent hip surgeries after PAO were collected from retrospective review of the medical records.
Table 1.
Demographics
| Demographic variables | Rectus-preserving | Control | p value | ||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 28 | 88% | 28 | 88% | > 0.9999 |
| Male | 4 | 13% | 4 | 13% | |
| Side | |||||
| Right | 19 | 59% | 17 | 53% | 0.7403 |
| Left | 13 | 41% | 15 | 47% | |
| Age (years)* | 22 | ± 8 | 23 | ± 7 | 0.5094 |
| BMI* | 23 | ± 4 | 24 | ± 5 | 0.1988 |
| Followup (months)* | 18 | ± 7 | 20 | ± 9 | 0.2405 |
* Mean ± SD.
Perioperative dependent variables of interest included operative blood loss (milliliters, estimated by Cell Saver® equipment [Haemonetics, Braintree, MA, USA] used for intraoperative blood collection and reinfusion) and preoperative and postoperative hematocrit and duration of surgery (from anesthesia records).
Hip pain and function outcomes were assessed by self-administered questionnaires (HHS) [7] collected preoperatively and postoperatively at minimum of 1 year followup.
Postoperative complications were classified by one of the authors (ENN) according to a modified Clavien-Dindo system that has been validated for hip preservation surgery [18]. The classification system includes five grades based on the type of treatment required and long-term morbidity of a complication. A Grade 1 complication requires no change in postoperative care, Grade 2 requires treatment on an outpatient basis, Grade 3 involves invasive (surgical or radiologic) procedures, Grade 4 includes potential life-threatening complications or complication with high morbidity, and Grade 5 complications involve death. This complication system was associated with high interrater (overall kappa = 0.887) and intrarater reliability (overall kappa = 0.891) [18] in a previous study.
Radiographic measurements were performed by a hip preservation surgeon not involved in the surgical care of the patients (ENN). The measurements were made on standing AP, lateral, and false-profile [10] pelvic radiographs obtained preoperatively and at the most recent clinic visit. The radiographic dependent variables of interest included the lateral center-edge angle of Wiberg [28], the acetabular roof obliquity angle of Tönnis [22], and the anterior center-edge angle of Lequesne and de Séze [10]. Radiographic grade of osteoarthritis was assessed using the Tönnis [22] grading scheme.
Surgical Technique
Surgery was performed by one of the senior authors (MBM, Y-JK) with the patient positioned supine on a radiolucent table and the entire ipsilateral lower extremity, including the hemipelvis draped free. Through a modified direct anterior approach [13], the tensor fasciae latae compartment is entered, and the tensor fasciae latae-sartorius interval is identified. The lateral femoral cutaneous nerve is not exposed and is protected in the sartorius fascia. The sartorius and inguinal ligament insertion are detached by a wafer osteotomy of the anterosuperior iliac spine.
In the classic technique (control group), the muscle fascia of the rectus femoris is opened longitudinally at the most lateral aspect of the muscle. The reflected head of the rectus femoris tendon is identified and transected (Fig. 1) and the direct head of the rectus is released from the anteroinferior iliac spine. The rectus tendon and muscle then are elevated distally and medially together with the iliocapsularis muscle from the joint capsule.
Fig. 1.
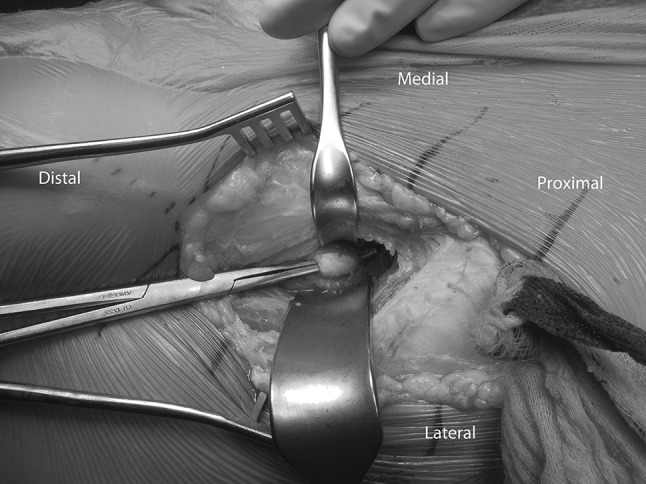
An intraoperative photograph shows a PAO using the classic approach. The indirect head of the rectus femoris tendon is identified and transected.
In the rectus-preserving approach, the iliacus muscle is identified proximally at the pelvic brim. The iliopsoas musculotendinous unit is dissected free from the periosteum of the inner table of the ilium and superior pubic ramus to allow exposure of the anatomic plane between the rectus femoris tendon and the iliacus muscle at the level of the anteroinferior iliac spine (Fig. 2). The muscle fascia of the rectus femoris is opened medially and the rectus femoris muscle belly is retracted laterally exposing the underlying iliocapsularis muscle. The iliocapsularis then is elevated from the joint capsule from lateral to medial, leaving the rectus tendon intact. It is important to release the iliocapsularis muscle insertion from the anteroinferior iliac spine and to retract it along with the iliopsoas muscle medially to allow for complete exposure of the interval between the iliopsoas and the joint capsule. From this point on, the procedure is performed the same way in both groups following a previously described technique [12]. The interval between the hip capsule and the iliopsoas tendon is dissected and enlarged sufficiently to allow the tip of a special angled chisel to be inserted onto the infracotyloid groove of the ischium for the first osteotomy. The next step is the osteotomy of the superior pubic ramus performed medial to the iliopectineal eminence. A supraacetabular iliac osteotomy is performed with an oscillating saw, beginning anteriorly just distal to the anterosuperior spine, angled posteriorly in the direction of the apex of the sciatic notch, stopping approximately 1 cm above the iliopectineal line. The posterior column osteotomy is performed next. A Schanz screw is inserted with a T-handle chuck in a predrilled hole at the level of the anteroinferior spine on the acetabular fragment and is used to help mobilize the fragment in the corrected position. The fragment is fixed provisionally with smooth 2.5 mm wires. After the acetabular position and passive hip flexion and internal rotation are checked clinically and under fluoroscopy, the acetabular fragment is fixed with either 3.5- or 4.5-mm cortical screws. An H-shaped anterior arthrotomy was performed in 94% of patients in the classic approach group. In one case, the labrum was degenerated and partially debrided, and in 18 (60%) patients, an osteochondroplasty of the femoral head-neck junction was performed to treat or prevent femoroacetabular impingement. If an arthrotomy is done, the capsule then is closed with interrupted sutures. With the classic approach the rectus femoris attachments are released, therefore the indirect and direct head are repaired after capsular closure, and the wound is sutured in layers. Opening of the hip was not performed in the rectus-sparing group. In 30 (93.7%) patients in the control group, an arthrotomy was performed. All patients received a standardized pain management protocol including an epidural catheter for the first 48 to 72 hours of the procedure with transitioning to oral pain medication as tolerated. Patients were discharged from the hospital once they could walk independently using two crutches with 20% to 30% of the body weight for weightbearing. Patients were instructed to walk with two crutches for the first 8 weeks after surgery. They then progressed to full weightbearing and started a progressive hip strengthening program with physical therapy. No patient(s) received prophylaxis for heterotopic ossification.
Fig. 2A–B.
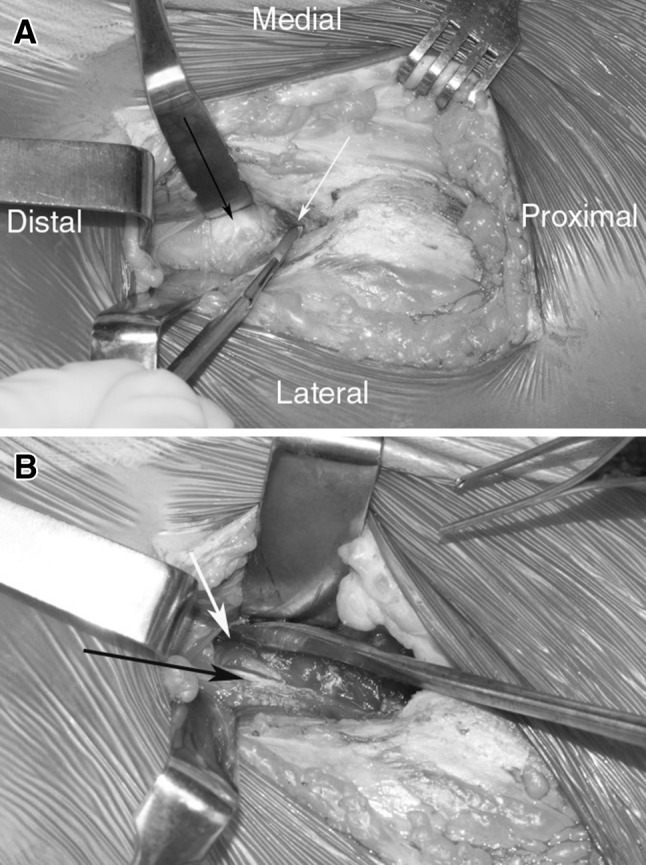
(A) An intraoperative photograph shows a PAO performed using the rectus-preserving approach. The rectus femoris tendon (black arrow) is identified and the rectus sheath is sharply entered medially to the tendon to allow dissection between the rectus and the iliacus (white arrow). (B) The rectus femoris (black arrow) is left intact. The iliopsoas has been retracted medially. The iliocapsularis muscle (white arrow) is identified and will be dissected medially from the hip capsule.
Statistical Analysis
Descriptive statistics were used to summarize the demographics and clinical characteristics associated with the treatment groups. Point estimates for the continuous variables were reported as the group mean and SD, when normally distributed, and as the group median and interquartile range when not normally distributed. Point estimates for the categorical variables were reported as the frequency (number) and percentage. Chi-square test, Fisher’s exact test, or Student’s t-test, when appropriate, was used to compare differences in the demographics and clinical characteristics between the groups. Independent sample t-tests were used to compare differences in estimated blood loss and hematocrit loss and operative time between the treatment groups. Because the blood loss was not normally distributed, a log transformation was applied to the blood loss variable. Chi-square or Fisher’s exact tests, when appropriate, were used to compare differences in the incidence of the different types of complications (lateral femoral cutaneous nerve dysesthesia, peroneal nerve dysesthesia, complications that required a change in postoperative care [Clavien-Dindo Grades 2–5 versus Grades 0–1]) and the need for subsequent hip surgery. Finally, multiple linear regression analyses were used to compare changes (postoperative score − preoperative score) in the HHS between the treatment groups. Preoperative HHS scores were controlled for in the linear regression model. Lateral center-edge angle, anterior center-edge angle, and Tönnis angle measurements were analyzed with generalized linear regression models. Main effects for time (preoperative versus postoperative), group (rectus sparing versus control), and their interaction (comparison of the amount of change achieved in the two groups after surgery) were included in the models. The unstructured covariance structure was used to account for within subject correlation owing to repeated measures (before and after surgery times). To facilitate future research efforts, post hoc power and sample size calculations were performed for all variables that were not significantly different between groups. The alpha level for all statistical tests was set at 0.05.
Results
Estimated surgical blood loss was an average of 4% (95% CI, −25% to 33%; p = 0.7844] greater in the control group compared with the rectus-preserving group but this difference did not reach statistical significance (Table 2). Based on a post hoc power analysis, our study has 5% power to detect a 4% difference in blood loss between groups. To achieve 80% power, future studies need to enroll 3163 subjects per group. Similarly, both groups had comparable hematocrit loss (mean difference, 1; 95% CI, −1 to 3; p = 0.3277). Based on a post hoc power analysis, the current study has 17% power to detect a significant difference between groups. A power group with a total of 247 subjects would provide 80% power to detect a difference of 1% between groups. The rectus-preserving approach did not decrease operative time in relation to the control group (mean difference, 15 minutes; 95% CI, −20 to 51; p = 0.3960). Based on a post hoc power analysis, the current study has 14% power to detect a significant difference between groups. A total of 329 subjects per group would provide 80% power to detect a difference of 15 minutes between groups.
Table 2.
Operative-related variables
| Operative variables | Rectus-preserving | Control | p value |
|---|---|---|---|
| Estimated blood loss (mL)* | 1000 (800–1600) | 1000 (700–1500) | 0.2405 |
| Hematocrit loss† | 7% ± 4% | 8% ± 4% | 0.3277 |
| Operative time (minutes)† | 270 ± 69 | 286 ± 70 | 0.396 |
* Median and interquartile range; †mean ± SD.
The HHS scores improved by an average of 25 points (95% CI, 21–29; p < 0.0001) in the rectus-preserving group and 21 points (95% CI, 17–25; p < 0.0001) in the control group. There was no difference (p = 0.1523) difference in the change in HHS scores that was achieved in each of the respective groups after PAO. Based on a post hoc power analysis, our study has 28% power to detect a significant difference. Assuming the same effect size, 116 subjects per group would provide 80% power to detect a significant difference between groups.
There were four complications that required treatment and changed postoperative course (Clavien-Dindo Grades 2–5) in the rectus-preserving group and eight in the control group (p = 0.2002) (Table 3). Based on a post hoc power analysis, the current study has 23% to detect a significant difference between groups at an alpha level of 0.05. Approximately 167 subjects per group would provide 80% power to detect a difference in complication rate of 23% between groups. Femoral nerve palsy was not observed in either group. There were two subsequent hip surgeries (Grade 3 complication) in each group. A 15-year-old boy with persistent groin pain after a rectus-preserving PAO underwent a revision PAO and an intertrochanteric femoral varus-derotational osteotomy. Two years after the index PAO, he was asymptomatic with a HHS of 99. A 26-year-old woman underwent hip arthroscopy for treatment of persistent hip pain associated with femoroacetabular impingement after a rectus-preserving PAO. Similarly, a 22-year-old woman underwent hip arthroscopy for femoroacetabular impingement (femoral head/neck osteochondroplasty) after a classic approach with arthrotomy. A 29-year-old woman in the control group had heterotopic ossification develop that was painful and limited hip flexion. She underwent excision of the heterotopic ossification.
Table 3.
Modified Clavien-Dindo grading of complication severity
| Complication grade | Rectus-preserving | Control | ||
|---|---|---|---|---|
| Grade 3 | ||||
| Secondary surgery for persistent pain | 2 | 6% | 1 | 3% |
| Symptomatic excision | 0 | 0 | 1 | 3% |
| Grade 2 | ||||
| Symptomatic heterotopic ossification | 0 | 0 | 1 | 3% |
| Lateral femoral cutaneous nerve dysesthesia | 0 | 0 | 1 | 3% |
| Fibrous union/stress reaction of the ischium | 1 | 3% | 2 | 6% |
| Sciatic/peroneal nerve dysesthesia | 1 | 3% | 2 | 6% |
| Grade 1 | ||||
| Asymptomatic heterotopic ossification | 1 | 3% | 9 | 28% |
| Lateral femoral cutaneous nerve dysesthesia | 17 | 53% | 19 | 59% |
| Grade 0 | ||||
| No complication | 13 | 41% | 6 | 19% |
There was no difference in the magnitude of radiographic correction achieved in the two groups regarding the lateral center-edge angle (p = 0.4463), Tönnis angle (p = 0.7953), or anterior center-edge angle (p = 0.0936) measurements (Table 4). There was no progression of Tönnis arthritis grade in either group. In the rectus-preserving group, the lateral center-edge angle improved (mean difference, 19°; 95% CI, 15°–22°; p < 0.0001) as did the Tönnis angle (mean difference, −14°; 95% CI, −17° to −11°; p < 0.0001) and the anterior center-edge angle (mean difference, 23°; 95% CI, 19°–27°; p < 0.0001) after surgery. Similarly, in the control group, the lateral center-edge angle improved (mean difference, 17°; 95% CI, 13°–20°; p < 0.0001) as did the Tönnis angle (mean difference, −14°; 95% CI, −17° to −10°; p < 0.0001) and the anterior center-edge angle (mean difference, 17°; 95% CI, 13°–22°; p < 0.0001) after surgery.
Table 4.
Preoperative and postoperative comparison of radiographic variables
| Variable | Presurgery | Postsurgery | ||||
|---|---|---|---|---|---|---|
| Rectus-preserving | Control | p value | Rectus-preserving | Control | p value | |
| ACEA | 3° (−21° to 25°) |
7° (−17° to 24°) |
0.2185 | 25° (13°–33°) |
24° (15°–32°) |
0.5807 |
| LCEA | 3° (−11° to 17°) |
3° (−30° to 17°) |
0.843 | 22° (9°–42°) |
20° (1°–36°) |
0.2566 |
| Tönnis angle | 22° (10°–38°) |
22° (6°–45°) |
0.8516 | 8° (−8° to 19°) |
9° (−10° to 39°) |
0.657 |
All values expressed as mean (range); ACEA = anterior center-edge angle; LCEA = lateral center-edge angle.
Discussion
The Bernese PAO has proven to be effective in reducing pain, improving function, and delaying the onset of osteoarthritis in symptomatic patients with hip dysplasia [6, 11, 15, 20, 25]. The PAO is a complex and extensive surgical procedure associated with a technical learning curve. Different types of surgical approaches influence PAO morbidity [8, 23, 27]. A transartorial less invasive technique has been reported as an alternative to the classic approach [24]. However, the limited exposure increases the technical complexity of surgery and may compromise the operative accuracy, negatively affect acetabular reorientation, and increase the risk of complications. In patients with no abnormality of the femoral head-neck junction, no signs of femoroacetabular impingement, and no labral tear observed on preoperative MR images, we have been preserving the rectus femoris tendon attachment in an effort to minimize soft tissue transection. However, to our knowledge, this rectus-preserving approach has not been compared with the classic approach to show its safety or efficacy. In this study, we therefore wished to compare this approach with the classic approach in terms of blood loss, operative time, functional and pain outcome assessed by the HHS, complications, and radiographic correction between a cohort with rectus-preserving PAOs and a cohort with the classic surgical approach transecting the rectus tendon.
We recognize several limitations of this study. First, we acknowledge a potential selection bias. We attempted to compensate for selection bias by designing a matched-cohort study. The rectus-preserving technique was indicated in a select group of patients who did not present with a history of catching or locking of the hip, no severe femoral head-neck junction deformity, no evidence of full-thickness labral tear on MR images, and a minimum 100° flexion and 20° internal rotation of the hip. Although the matching process controls for age, sex, BMI, and diagnosis, the control group includes patients for whom hip arthrotomies were routinely performed. However, intraarticular work was performed in only 60% of these patients after the joint was opened. Adding an arthrotomy potentially could add to the length of surgery and blood loss which was not observed in the study. Additional studies are necessary to identify patients who benefit from arthrotomy during a PAO to avoid unnecessary violation of the joint. Second, there is a potential for assessment bias for evaluation of the classification of complications and measurement of radiographs. However the complication scheme [18] and the radiographic parameters [21] used in our study have been reported to have good intraobserver and interobserver reliability. Third, because the study was retrospective there is concern for transfer bias. All patients included in the study had more than 1 year followup, however some patients had longer followup than others and some have not been evaluated in the past 2 years. Fourth, although we detected no differences in blood loss, complication rate, HHS at 1 year, and radiographic measurement, the number of patients included limited the power to detect such differences. However, we believe our study is important because it established comparable safety and accuracy between the rectus-preserving and the classic approaches. Future research is needed to determine whether preserving the rectus femoris tendon attachment affects functional recovery including hip flexion strength. Finally, the retrospective study design may have limited our ability to identify complications in both groups. For these reasons we chose to compare complications requiring treatment (complications Clavien-Dindo Grades 2–5) in both groups which are less likely to be under-registered in medical charts. The complication rate we reported was lower in both groups when compared with rates reported in a systematic literature review of PAO of 6% to 37% rates of major complications [4].
We observed no difference in the blood loss and operative time between the rectus-preserving group and the classic approach group. Longer surgical time has been shown to correlate with blood loss during PAO [9].Operative time and blood loss were significantly lower when a minimally invasive transartorial approach was used compared with the extensive ilioinguinal approach [23]. Previous studies estimated intraoperative PAO blood loss from 300 mL to 4500 mL [2, 6, 16]. In the original description of the technique, Ganz et al. [6] reported an average of 3 L of blood loss during the early part of their series with improvement to an average of 800 mL in the last 10 patients in the series. Although surgeon(s)’ experience had a direct effect on rate of complications and operative time, it was not a factor determining blood loss, transfusion requirement, and hospital stay in an investigation comparing one surgeon’s experience with his first 35 cases and subsequent 35 cases [5]. Trumble et al. [27] reported an average operative time of 6.5 hours with the ilioinguinal approach versus 4.5 hours when a modified Smith-Petersen approach was used. The average blood loss was 1400 mL compared with 800 mL, respectively.
In our patients, there was no difference in pain and functional improvement assessed by the HSS between the rectus-preserving approach (mean improvement of 25 points) and the classic approach (mean improvement of 21 points) after1 year postoperative. Several studies have reported pain relief, improved hip function, and activity level at short- to long-term followup after PAO using the classic approach [4, 6, 11, 15, 16, 20, 25, 27]. In a previous systematic literature review of PAO outcomes, Clohisy et al. [4] reported on eight studies of classic-approach PAOs that used the HHS as an outcome measure, with mean improvement ranging from 14.5 to 33 points. We are aware of only two studies reporting a PAO technique without complete transection of the rectus femoris tendon attachment. Siebenrock et al. [17] reported their current PAO technique using an ilioinguinal approach and preserving the rectus femoris tendon attachment. However, their study was a review article that did not report any clinical, functional, or radiographic results nor did they compare the technique with the classic approach. Troelsen et al. [24] compared the ilioinguinal approach with a less invasive transartorial approach that preserves the rectus femoris attachment. Although they reported no differences in short-term hip survival rates without conversion to hip arthroplasty, there were no specific hip pain and functional outcomes in their study.
In our series, both groups had an acceptable rate of major complications when compared with rates reported in other studies [4–6, 12, 16, 20, 25, 27]. The frequency of complications requiring treatment (Clavien-Dindo Grades 2 and 3) in both groups was in line with a systematic literature review of PAO, which reported a 6% to 37% rate of major complications [4]. This may reflect the authors’ advanced experience with this procedure as the rate of complications after PAO has been attributed to a long learning curve associated with mastering the surgical technique. Although there may be concern related to compromising the innervation of the rectus femoris by dissecting medially to the muscle, we did not find any case of femoral nerve dysfunction after PAO in the rectus-preserving group. Although we found no difference in lateral femoral cutaneous nerve dysesthesia between the two groups, the rate of lateral femoral cutaneous nerve dysesthesia in our patients is slightly greater than the range of 1.5% to 38% previously reported [4]. This complication, however, has been reported to be common with some calling it “trivial” after PAO [5]. One patient from each group underwent a secondary hip arthroscopy for treatment of persistent pain associated with femoroacetabular impingement. Femoroacetabular impingement after reorientation of the acetabulum is recognized as a potential cause of PAO failure [1, 14]. However, it is not clear from the current literature, which patients benefit from an arthrotomy and correction of femoral head-neck offset at the time of PAO. Albers et al. [1] suggested that hips with a spherical femoral head present before surgery or after femoral head-neck osteochondroplasty through an arthrotomy at the time of PAO had a higher survivorship at 10 years followup when compared with hips with morphologic features of femoroacetabular impingment.
We found no difference in the severity of hip dysplasia between our two groups as assessed by the lateral center-edge angle, anterior center-edge angle, Tönnis angle, and grade of arthritis before surgery. Preserving the rectus femoris tendon did not compromise correction of acetabular dysplasia and improvement of femoral head coverage. In both groups, there was significant improvement in the radiographic parameters similar to those reported in previous studies [4, 8, 11, 13, 15–17, 20, 23–26]. In contrast, Troelsen et al. [23] reported better reorientation of the acetabulum assessed by the lateral center-edge angle and Tönnis angle when a less invasive transartorial approach was compared with an ilioinguinal approach.
Patients who received the rectus-preserving technique had similar blood loss, operative time, complication patterns, HHS, and radiographic correction of acetabular dysplasia at short-term followup when compared with patients who had the classic approach. The ideal patient for the rectus-preserving approach would have minimal or no proximal femur head-neck deformity, hip flexion greater than 100°, and internal rotation greater than 20°, with no full-thickness labral tear observed on preoperative MR images. Additional investigation is necessary to determine whether preserving the rectus femoris tendon attachment yields functional benefits such as improving hip flexor strength after PAO.
Acknowledgments
We thank Kerry Murray PA-C (Department of Orthopaedics, Children’s Hospital Boston), and Gloria Boye BA (Department of Orthopaedics, Children’s Hospital Boston) for contributions to data acquisition for this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Boston Children’s Hospital, Boston, MA, USA.
References
- 1.Albers CE, Steppacher SD, Ganz R, Tannast M, Siebenrock KA. Impingement adversely affects 10-year survivorship after periacetabular osteotomy for DDH. Clin Orthop Relat Res. 2013;471:1602–1614. doi: 10.1007/s11999-013-2799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atwal NS, Bedi G, Lankester BJ, Campbell D, Gargan MF. Management of blood loss in periacetabular osteotomy. Hip Int. 2008;18:95–100. doi: 10.1177/112070000801800205. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Defining overweight and obesity. Available at: http://www.cdc.gov/obesity/defining.html. Accessed March 28, 2014.
- 4.Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467:2041–2052. doi: 10.1007/s11999-009-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:33–37. doi: 10.1097/00003086-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias: technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36. [PubMed] [Google Scholar]
- 7.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 8.Hussell JG, Mast JW, Mayo KA, Howie DW, Ganz R. A comparison of different surgical approaches for the periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:64–72. [PubMed] [Google Scholar]
- 9.Lee CB, Kalish LA, Millis MB, Kim YJ. Predictors of blood loss and haematocrit after periacetabular osteotomy. Hip Int. 2013;23(suppl 9):S8–S13. doi: 10.5301/hipint.5000062. [DOI] [PubMed] [Google Scholar]
- 10.Lequesne M, de Sèze S. [False profile of the pelvis: a new radiographic incidence for the study of the hip. Its use in dysplasias and different coxopathies][in French] Rev Rhum Mal Osteoartic. 1961;28:643–652. [PubMed] [Google Scholar]
- 11.Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91:2113–2123. doi: 10.2106/JBJS.G.00143. [DOI] [PubMed] [Google Scholar]
- 12.Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the bernese periacetabular osteotomy and predictors of clinical outcome: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 pt 2):115–129. doi: 10.2106/JBJS.J.00646. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SB, Millis MB. Periacetabular osteotomy without abductor dissection using direct anterior exposure. Clin Orthop Relat Res. 1999;364:92–98. doi: 10.1097/00003086-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:93–99. doi: 10.1097/00003086-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471:981–988. doi: 10.1007/s11999-012-2578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239–245. [PubMed] [Google Scholar]
- 17.Siebenrock KA, Steppacher SD, Albers CE, Haefeli PC, Tannast M. Diagnosis and management of developmental dysplasia of the hip from triradiate closure through young adulthood. J Bone Joint Surg Am. 2013;95:748–755. doi: 10.2106/00004623-201304170-00012. [DOI] [PubMed] [Google Scholar]
- 18.Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470:2220–2226. doi: 10.1007/s11999-012-2343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith-Petersen MN. Approach to and exposure of the hip joint for mold arthroplasty. J Bone Joint Surg Am. 1949;31:40–46. [PubMed] [Google Scholar]
- 20.Steppacher SD, Tannast M, Ganz R, Siebenrock KA. Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop Relat Res. 2008;466:1633–1644. doi: 10.1007/s11999-008-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannast M, Mistry S, Steppacher SD, Reichenbach S, Langlotz F, Siebenrock KA, Zheng G. Radiographic analysis of femoroacetabular impingement with Hip2Norm-reliable and validated. J Orthop Res. 2008;26:1199–1205. doi: 10.1002/jor.20653. [DOI] [PubMed] [Google Scholar]
- 22.Tonnis D. General radiograph of the hip joint. In: Tonnis D, ed. Congenital Dysplasia, Dislocation of the Hip. New York, NY:Springer; 1987:100–142.
- 23.Troelsen A, Elmengaard B, Soballe K. Comparison of the minimally invasive and ilioinguinal approaches for periacetabular osteotomy: 263 single-surgeon procedures in well-defined study groups. Acta Orthop. 2008;79:777–784. doi: 10.1080/17453670810016849. [DOI] [PubMed] [Google Scholar]
- 24.Troelsen A, Elmengaard B, Soballe K. A new minimally invasive transsartorial approach for periacetabular osteotomy. J Bone Joint Surg Am. 2008;90:493–498. doi: 10.2106/JBJS.F.01399. [DOI] [PubMed] [Google Scholar]
- 25.Troelsen A, Elmengaard B, Soballe K. Medium-term outcome of periacetabular osteotomy and predictors of conversion to total hip replacement. J Bone Joint Surg Am. 2009;91:2169–2179. doi: 10.2106/JBJS.H.00994. [DOI] [PubMed] [Google Scholar]
- 26.Trousdale RT, Ekkernkamp A, Ganz R, Wallrichs SL. Periacetabular and intertrochanteric osteotomy for the treatment of osteoarthrosis in dysplastic hips. J Bone Joint Surg Am. 1995;77:73–85. doi: 10.2106/00004623-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Trumble SJ, Mayo KA, Mast JW. The periacetabular osteotomy: minimum 2 year followup in more than 100 hips. Clin Orthop Relat Res. 1999;363:54–63. doi: 10.1097/00003086-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint: with special reference to the complication of osteoarthritis. Acta Chir Scand. 1939;83:28–38. [Google Scholar]


