Abstract
Objective: To explore the relationship between methylation of interferon gamma (IFN-γ) gene and tumorigenesis in cervical cancer tissues, the biopsy specimens of cervical cancer and cervical intraepithelial neoplasia (CIN) (I-III) patients as well as normal controls were collected and analyzed. Methods: The methylation of the IFN-γ gene was verified by using methylation-specific PCR and DNA sequencing analysis, and the expression levels of IFN-γ mRNA were detected using quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR). Results: The methylation rates of the IFN-γ gene were significantly higher in cervical cancer tissues (15/43, 34.9%) than those in CIN (3/23, 13.0% of CIN I; 6/39, 15.4% of CIN II/III) and normal cervical tissues (2/43, 4.7%) (P < 0.01). Furthermore, the mRNA expression of IFN-γ in cervical tumors with methylation (0.71 ± 0.13, n = 8) was lower than that in those without methylation (1.58 ± 0.32, n = 27) (P < 0.05). Likewise, the IFN-γ expression levels in CIN II/III tissues with methylation (0.87 ± 0.16, n = 5) were significantly (P < 0.01) lower compared to those without methylation (2.12 ± 0.27, n = 32). Conclusion: The hypermethylation of IFN-γ gene may be related with tumorigenesis of cervical cancer.
Cervical cancer is ranked as the second most common cancer in women worldwide, and continues to be a major public health problem1. Immunological imbalance created by infiltrating inflammatory cells may contribute to cancer growth and spread of cervical carcinogenesis2. The cytokine network of several common tumours is rich in inflammatory cytokines, growth factors, and chemokines3. Now there are evidences that inflammatory cytokines and chemokines, which can be produced by the tumour cells and/or tumour-associated leucocytes and platelets, may contribute directly to malignant progression. Examples are tumour necrosis factor-alpha (TNF-α), IL-1 and 6, and Interferon gamma (IFN-γ). The local production of cytokines within the tumor microenvironment can prevent the effector's response4 and cytokines also can mediate the activities of immune cells in the fight against malignant cells5.
IFN-γ is a pleiotropic cytokine secreted by type-1 helper (Th1) T cells, cytotoxic T cells, and stimulated natural killer cells in response to antigenic stimulation and involved in activation of macrophages and endothelial cells6. Production of IFN-γ is related to the induction of reaction in T lymphocytes, which contributes to enhance an immune response against malignant cells.
DNA methylation is characterized by the addition of methyl groups in cytosines within cytosine-phosphate-guanine (CpG) islands. Unmethylated islands are related with transcriptionally active regions, whereas methylated DNA recruits methyl-binding proteins that promote chromatin compaction and prevent the binding of transcription factors7,8. Recently, evidence is emerging about the importance of epigenetic regulatory mechanisms in the control of inflammatory response9. Changes in DNA methylation patterns, particularly in the promoter region of genes, can have profound effects on gene expression10.
Epigenetic changes occur in cytokine genes in human cells, affecting the ability of the cell to express cytokines11. Methylation profiling of cancer cells and studies of individual genes disclose that gene-specific hypomethylation occurs frequently in diverse cancers, e.g., colon cancer12 and breast cancer13. Furthermore, hypomethylation of specific genes correlates well with increased transcription levels13. Although CpG methylation in the IFN-γ promoter is considered a negative transcriptional regulator of IFN-γ production14, such events have not been investigated in cervical tissues yet. Therefore, the aim of the present study was to evaluate the methylation status of the IFN-γ promoter region in cervical cancer tissues compared to normal and cervical intraepithelial neoplasia (CIN) tissues.
Methods
Clinical samples and DNA extraction
Human tissue samples were obtained from 148 patients (43 normal cervical tissues, 62 CIN tissues (CIN I: 23 cases; CIN II/III: 39 cases) and 43 cervical squamous carcinomas tissues) from January 2012 to Octomber 2013. Cervical tissues were gained by trans-vaginal biopsies and then flash-frozen in liquid nitrogen and stored at -150°C. All of these patients have been informed consent before collection of their samples, according to institutional guidelines. This protocol was approved by a regional ethics committee, in Tangshan of Hebei province, China. After detection, they underwent surgical resection of primary cervical cancer at the Department of Obstetrics and Gynecology inTangshan workers hospital. The histological type and grade of tumor were classified on the basis of WHO criteria. The stage of each cancer was established according to the International Federation of Gynaecology and Obstetrics (FIGO) criteria. These tissue samples for CIN diagnosis were performed by using micro-excision. All primary tumor tissues and control samples were diagnosed by HE-stained. The frozen samples were used for genomic DNA extraction from tissues using the standard Proteinase K treatment followed by phenol/chloroform extraction. The concentration of DNA was determined with a spectrophotometer.
Bisulfite Modification
Methylation pattern of tissues were assessed using DNA modification by bisulfite treatment similar to that reported by Goldenberg et al15. With the bisulfite treatment, unmethylated cytosines of DNA were converted to uracil, whereas methylated cytosines remained unmodified. Extracted genomic DNA was modified using bisulfite conversion kit (EZ DNA Methylation-Gold Kit™; ZYMO Research Corp.). The modified DNA was ready for immediate analysis or could be stored at or below −20°C for later use. For long term storage, it should be stored at or below −70°C. The volume of 1 μl eluted DNA was used for each PCR.
Methylation-specific PCR (MSP)
Methylation-specific polymerase chain reaction are distinguished from the presence of methylation based on alterations produced after bisulfite treatment of DNA using specific primers for methylated or unmethylated DNA. All the samples were analyzed by MSP. The Primer sequences, annealing temperatures, and the expected product sizes were listed in Table 1.
Table 1. Primer sequences, annealing temperatures, and PCR product sizes.
| Gene | Sequence (5′ -3′) | Location | Annealing temperature (°C) | Product size (bp) | |
|---|---|---|---|---|---|
| IFN-γ | Uf | TGAAGAGTTAATATTTTATTAGGGTGA | −320 ~ −164 | 60 | 156 |
| Ur | TAAACTCCTTAAATCCTTTAACAAT | ||||
| Mf | AAGAGTTAATATTTTATTAG GGGGA | −317 ~ −164 | 59 | 154 | |
| Mr | TAAACTCCTTAAATCCTTTAAGGAT |
GenBank accession no.: J00219.
Polymerase chain reaction (PCR) was performed in a total volume of 25 μL containing 2.5 μL 10×PCR buffer, 5 μL dNTP-mix (1 mmol/L of each), 0.75 μL of magnesium chloride (50 mmol/L), 0.5 μL of each primer (20 pmol/mL), 2.5 units of Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA), and 200 ng of DNA. The PCR conditions were 95°C for 3 min and then, 35 cycles of 94°C for 45 s, 60°C for 20 s, and 72°C for 45 s, with a final extension at 72°C for 5 min. The reactions were performed into a thermocyler (Eppendorf AG, Hamburg, Germany). An untreated blood DNA from a normal individual was used as negative control. A methylation-positive DNA control was made in vitro using SssI methylase (New England Biolabs, Beverly, MA) which methylated every cytosine of CpG dinucleotide in the DNA. Water blanks were included with each assay. The same PCR conditions were used for tumor, CIN, and normal tissue DNA. PCR products were visualized on 2% agarose gels stained with ethidium bromide.
Positive amplification only for unmethylated primers was interpreted as unmethylation. Positive amplification only for methylated primers or for both methylated and unmethylated primers were considered as methylation.
Direct sequencing
Methylated and unmethylated PCR products were confirmed by direct sequencing. PCR products were gel purified, and analyzed on an automated DNA sequence analyser (ABI 3730xl, Life Technologies, Company) using BigDye® Terminator kit (Life Technologies, Company).
RNA Extraction and Quantitative Real-time PCR
Total RNA was extracted using Trizol reagent, and the RNA obtained was treated with RNAse-free DNAase I (Invitrogen Life Technologies) according to the manufacturer's protocol. The complementary DNA was synthesized from 1 μg of total RNA using superscript first-strand synthesis system (Invitrogen Life Technologies). Real-time PCR was performed using SYBR-green fluorescence quantification system in a Step-One real-time PCR 96-well plate (Applied Biosystems, Warrington, UK). Each cDNA sample (2.5 μl) was used as a template for the PCR amplification mixture containing forward and reverse primers (900 mM each). Reaction mixtures were subjected to the following amplification scheme: one cycle at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 56°C for 1 min. Relative gene expression was calculated using the 2-ΔΔCt method, and the expression data were normalized with endogenous β-actin. The sequences of primers used for IFN-γ were 5′ -GGCATTTTGAAGAATTGGAAAG-3′ (forward) and 5′ -TTTGGATGCTCTGGTCATCTT-3′ (reverse) as previously described16.The primers used for β-actin were 5′-TGCCGACAGGATGCAGAAG-3′ (forward) and 5′ -CTCAGGAGGAGCAATGATCTTGA-3′ (reverse) as previously described17. All real-time PCRs were performed in triplicate.
Statistical Analysis
A Student t-test and an analysis of variance (ANOVA) were used to detect differences in the mean values of the variables. Fisher's exact test was used appropriately to analyze differences in the rate of each variable. P < 0.05 was taken as significant. All the statistical analyses were performed with the software SPSS 13.0.
Results
Aberrant promoter methylation of IFN-γ
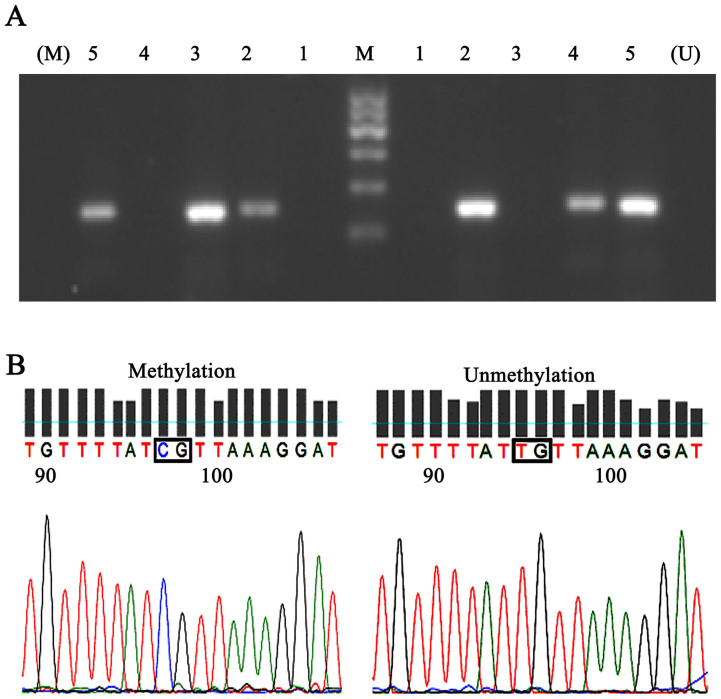
Aberrant promoter methylation of IFN-γ was shown in Table 2 and representative bands of the MSP reactions of IFN-γ gene were presented in Fig. 1 A. Accordingly, there was no significant difference in age among three groups (P = 0.367) (Table 2). The frequency of IFN-γ promoter methylation was 34.9% in cervical cancer, 13.0% in CIN I, 15.4% in CIN II/III, and 4.7% in normal cervical tissues. The methylation rates of the IFN-γ gene promoter were significantly higher in cervical cancer tissues than those in CIN and normal tissues (P = 0.003) (Table 2).
Table 2. The methylation of the IFN-γ gene in normal cervical tissue, CIN and cervical cancer.
| Group | Age (years old) | Methylation (n) | Methylation rate (%) |
|---|---|---|---|
| Control (n = 43) | 42.67 ± 10.61 | 2 | 4.7 |
| CIN I (n = 23) | 43.32 ± 8.54 | 3 | 13.0 |
| CIN II/III (n = 39) | 42.97 ± 9.48 | 6 | 15.4 |
| Cancer (n = 43) | 44.39 ± 10.83 | 15 | 34.9 *, #, & |
| P-Value | 0.367a | 0.003b | |
a. ANOVA test, b. Fisher's exact test. Compared with control group, *P < 0.05; compared with CIN I group, #P < 0.05; compared with CIN II/III group, &P < 0.05.
Figure 1. Representative data of the PCR products and the sequencing of IFN-γ gene.
(A) The MSP results of the IFN-γ gene. Shown are representative bands. U shows PCR products when amplified by unmethylated primers and M shows PCR products when amplified by methylated primers. Lane 1 shows a negative control. Lane 2 and 5 shows samples with partial methylation. Lane 3 shows samples with total methylation. Lane 4 shows an unmethylated sample. (B) Sequencing of the IFN-γ gene with representative data of CpG with Unmethylation and Methylation.
DNA Sequencing of MSP Products
The DNA sequencing was carried out in samples of the control, CIN I, CIN II/III and cervical cancer groups, respectively. Sequencing of the MSP products from the methylated control DNA showed the expected nucleotide changes. Representative results of bisulfite sequence analysis for IFN-γ gene promoter were shown in Fig. 1 B. There was only one CpG site in the amplicon excluding the primer sites.
Transcriptional activation by IFN-γ methylation in the cervical tissues
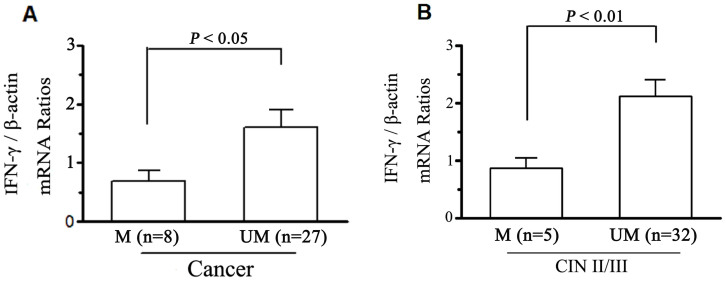
The mRNA expressions of IFN-γ promoter methylation on transcriptional silencing in control, CIN I, CIN II/III, and cervical cancer tissues were detected by real-time RT-PCR. The loss of IFN-γ expression was present 1 of 43 (2.3%) in Control, 2 of 23 (8.7%) in CIN I, 2 of 39 (5.1%) in CIN II/III, and 8 of 43 (18.6%) in cancer, respectively (Tab. 3). IFN-γ mRNA levels in cervical tumors with methylation (0.71 ± 0.13, n = 8) were significantly (P < 0.05) lower than those in cervical tumors unmethylated (1.58 ± 0.32, n = 27), and IFN-γ mRNA levels in CIN II/III tissues with methylation (0.87 ± 0.16, n = 5) were significantly (P < 0.01) lower than those unmethylated (2.12 ± 0.27, n = 32) (Fig. 2).
Table 3. Relationship between expression and methylation of IFN-γ in cervical tissues.
| IFN-γ mRNA Expression | Methylation status | Control (n = 43) | CIN I (n = 23) | CIN II/III (n = 39) | Cancer (n = 43) |
|---|---|---|---|---|---|
| Present | Methylated | 1 | 1 | 5 | 8 |
| Unmethylated | 42 | 20 | 32 | 27 | |
| Absent | Methylated | 1 | 2 | 1 | 7 |
| Unmethylated | 0 | 0 | 1 | 1 |
Figure 2. CpG methylation and mRNA expression of the IFN-γ gene in cervical tissues.
IFN-γ mRNA expression were detected using quantitative realtime RT-PCR. The quantified value was defined as the ratio of the IFN-γ PCR products compared with those of the β-actin. Columns represent mean value of IFN-γ mRNA levels of cervical cancer (A) and CIN II/III (B). M and UM represent methylation and unmethylation respectively.
Clinicopathological features and hypermethylation of IFN-γ in cervical cancer
Based on the methylation status, the results of mutiple variable analysis of the IFN-γ promoter regions in cervical cancer tissues were shown in Table 4. The clinicopathological parameters were evaluated including nodal status, squamous carcinomas pathology classification and clinical stages. There were no obvious relationship between methylation of IFN-γ and the following prognostic factors—nodal status (P = 0.539), squamous carcinomas pathology classification (P = 0.237), and clinical stages (P = 0.396).
Table 4. Correlation of clinicopathological factors with the methylation status of the IFN-γ gene in cervical cancer tissues.
| Patients with methylated | Patients with unmethylated | ||
|---|---|---|---|
| IFN-γ (%) (n = 15) | IFN-γ (%) (n = 28) | P value | |
| Nodal metastasis | |||
| Positive | 12 (80.0) | 20 (71.4) | 0.539 |
| Negative | 3 (20.0) | 8 (28.6) | |
| Squamous carcinomas Pathology classification | |||
| High | 7 (46.7) | 7 (25.0) | 0.237 |
| Middle | 3 (20.0) | 12 (42.9) | |
| Low | 5 (33.3) | 9 (32.1) | |
| Clinical stages (FIGO,2000) | |||
| I stage | 6 (40.0) | 15 (53.6) | 0.396 |
| II/III stage | 9 (60.0) | 13 (46.4) |
Discussion
In the present study, the methylation patterns of the IFN-γ gene in cervical tissues from normal controls, CIN and cervical cancer patients were detected. These data provided evidence that IFN-γ methylation was higher in cervical cancer tissue than in the normal and CIN groups, and DNA hypermethylation in the promoter region may influence gene activation during cervical tumorigenesis.
IFN-γ exhibits both antitumor and protumor activities18. The dual opposing functions of immunity formed conceptual basis for cancer immunosurveillance, equilibrium and escape, also named cancer immunoediting19. In this process, many immune cells might interact with tumour cells from the earliest stages of transformation to the terminal phase of widespread metastasis, in which IFNs have been found to have distinct functions. Vijay Shankaran, et al20 found that lymphocytes and IFN-γ collaborate to protect against development of carcinogen-induced sarcomas and spontaneous epithelial carcinomas and also to select for tumor cells with reduced immunogenicity. Therefore, IFN-γ has been shown to be crucial components of the cancer-immunoediting process, and controls the immunogenicity of tumour cells, possibly as a result of the selective production of IFN-γ in the tumor microenvironment21.
IFN-γ is a major contributor to an effective Th1-type cellular immune response against HPV infection22, and nearly all cells constitutively express functional IFN receptors. Many studies shown that a wide complement of immune cells can be found in the cervical epithelium, including T cells (CD4+ and CD8+ T cells, major sources of IFN-γ)23,24. Decreased expression of IFN-γ results in suppression of cell-mediated local immune responses and enhances persistent high-risk HPV infection of the uterine cervix, which may promote HPV invasion and tumor progression25,26. Reduced epithelial and sub-epithelial IFN-γ, may play a role in the development and progression of HPV16 associated cervical pre-cancer27. The role of IFN-γ has been studied extensively and there was evidence that intralesional IFN-γ might be a prognostic marker for clearance of high-risk HPV28.
The existence of a cancer-immunosurveillance process illustrate that suppression of tumour growth might be mediated by extrinsic forces. Epigenetic modification of gene expression is a novel mechanism by which environmental exposures may influence disease expression through modification of promoter regions regulating gene transcription. Recent evidence indicates that methylation patterns changes could occur in cervical cancer. The interaction between DNA methylation and inflammation may have relevance for analyzing the effect of inflammation on tumor development. As a naïve CD4+ T cell develops into a Th1 cell that secretes predominantly IFN-γ, the expression or permanent silencing of one cytokine gene is orchestrated using epigenetic mechanisms29. Activated Ag-specific CD8 T cells exhibit rapid DNA demethylation at IFN-γ loci and substantial histone acetylation at the IFN-γ promoter and enhancer regions, which occur early after HPV infection at the effector stage. However, activated unhelped CD8 T cells, which fail to develop into functional memory and are incapable of rapid cytokine production, exhibit increased DNA methylation at the IL-2 promoter and fail to acetylate histones at the IFN-γ locus30. This study suggested that most cervical cancer samples exhibited hypermethylation of IFN-γ in contrast to control samples. This indicates that cervical tumorigenesis may be associated with DNA hypermethylation in the promoter region of the IFN-γ gene.
To verify the hypothesis of epigenetic regulation of IFN-γ production, the transcription of this gene in each group was evaluated according to the MSP status. Although loss of IFN-γ expression was present in 1 of 43 (2.3%) in Control, 2 of 23 (8.7%) in CIN I, 2 of 39 (5.1%) in CIN II/III, and 8 of 43 (18.6%) in cancer), the methylated samples with observed gene expression showed lower mRNA levels of IFN-γ than unmethylated samples in CIN II/III and cancer groups. There was only one methylated sample present in control and CIN?tissues respectively, and gene expressions were also low in control (0.77) and CIN I (0.62) tissues. These data would suggest that decreased production of IFN-γ via hypermethylation and silencing of its gene promoter could enhance Th2 differentiation and Th2 cytokine-directed inflammation and persistence infection of high-risk HPV, which could be second or/and third phase of cancer-immunosurveillance process. Although we did not investigate the entire epigenome in this study, it is probable that epigenetic modification of multiple genes could also be associated with cervical tumorigenesis.
The methylation of IFN-γ had no obvious association with some prognostic variables including age, nodal status, squamous carcinomas pathology classification and clinical stages of cervical cancer. However, IFN-γ methylation demonstrated a significant correlation with the lower expression of IFN-γ.
The present study suggests that methylation induced silencing of IFN-γ gene may play an important role in the pathogenesis of cervical cancer. In future studies, methylation status of more cytokines should be further investigated to identify the relationships between their methylation status and cervical tumorigenesis. What's more, the critical roles need to be recognized which are played by the immune system in cervical oncogenesis, progression and therapeutic response.
Author Contributions
D.M. and C.Y.J. wrote the main manuscript text. X.L.H. prepared figures 1-2 and tables 1-4. All authors reviewed the manuscript.
Acknowledgments
This work was supported by grants from the Medical Science and Technology Development and Research Center of China's Ministry of Health (Nos. W2013GJO3), and the Nature Science Foundation of Tangshan city, Hebei province (Nos. 12140209A-10).
References
- Arbyn M., Castellsague X., De Sanjose S. et al. Worldwide burden of cervical cancer in 2008. Ann Oncol 22 (12), 2675–2686 (2011). [DOI] [PubMed] [Google Scholar]
- Jacobs N., Giannini S. L., Doyen J. et al. Inversemodulation of IL-10 and IL-12 in the blood of women with preneoplastic lesions of the uterine cervix. Clin Exp Immunol 111, 219–224 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M. J., Cretney E., Kershaw M. H. et al. Cytokines in cancer immunity and immunotherapy. Immunol Rev 202, 275e93 (2004). [DOI] [PubMed] [Google Scholar]
- Venetsanakos E., Beckman I., Bradley J. et al. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Brit J Cancer 75(12), 1826–1830 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S., Wang E. & Marincola F. M. Cytokines and immune response in the tumor microenvironment. J Immunother 24(5), 392–407 (2001). [PubMed] [Google Scholar]
- Woodman J. P., Dimier I. H. & Bout D. T. Human endothelial cells are activated by IFN-gamma to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol 147(6), 2019–2023 (1991). [PubMed] [Google Scholar]
- Jones P. L., Veenstra G. J., Wade P. A. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19, 187–191 (1998). [DOI] [PubMed] [Google Scholar]
- Egger G., Liang G., Aparicio A. et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463 (2004). [DOI] [PubMed] [Google Scholar]
- Wilson A. G. Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol 79(8S), 1514–1519 (2008). [DOI] [PubMed] [Google Scholar]
- Gopissetty G., Ramachandram K. & Singal R. DNA methylation and apoptosis. Mol. Immunol 43, 1729–1740 (2006). [DOI] [PubMed] [Google Scholar]
- Sanders V. M. Epigenetic regulation of Th1 and Th2 cell development. Brain Behav Immun 20, 317–324 (2006). [DOI] [PubMed] [Google Scholar]
- Frigola J., Sole X., Paz M. F. et al. Differential DNA hypermethylation and hypomethylation signatures in colorectal cancer. Hum Mol Genet 4(2), 319–326 (2005). [DOI] [PubMed] [Google Scholar]
- Feinberg A. P. & Tycko B. The history of cancer epigenetics. Nat Rev 4(2), 143–153 (2004). [DOI] [PubMed] [Google Scholar]
- White G. P., Watt P. M., Holt B. J. et al. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol 168, 2820-2827 (2002). [DOI] [PubMed] [Google Scholar]
- Goldenberg D., Harden S., Masayesva B. G. et al. Intraoperative molecular margin analysis in head and neck cancer. J Am Med Assoc 130(1), 39–44 (2004). [DOI] [PubMed] [Google Scholar]
- Dutzan N., Vernal R., Hernandez M. et al. Levels of interferon- gamma and transcription factor T-bet in progressive periodontal lesions in patients with chronic periodontitis. J Periodontol 80(2), 290–296 (2009). [DOI] [PubMed] [Google Scholar]
- Cardoso F. P., Viana M. B., Sobrinho A. P. R. et al. Methylation Pattern of the IFN-g Gene in Human Dental Pulp. J Endodont 36(4), 642–646 (2010). [DOI] [PubMed] [Google Scholar]
- Zaidi M. R. & Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res 17(19), 6118–6124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. P., Koebel C. M. & Schreiber R. D. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 6(11), 836–848 (2006). [DOI] [PubMed] [Google Scholar]
- Shankaran V., Ikeda H., Bruce A. T. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410(6832), 1107–1111 (2001). [DOI] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R. & Karin M. Immunity, inflammation, and cancer. Cell 140(6), 883–899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C. & Kurman R. J. Analysis of cytokine profiles in patients with human papillomavirus-associated neoplasms. J Natl Cancer Inst 89(3), 185–187 (1997). [DOI] [PubMed] [Google Scholar]
- Johansson E. L., Rudin A., Wassén L. et al. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology 96, 272–277 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J., Quayle A. J. & Anderson D. J. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 73, 1253–1263 (2005). [DOI] [PubMed] [Google Scholar]
- Scott M., Stites D. P. & Moscicki A. B. Th1 cytokine patterns in cervical human papillomavirus infection. Clin Diagn Lab Immun 6(5), 751–755 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M., Nakagawa M. & Moscicki A. B. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immun 8(2), 209–220 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- M El-Sherif A., Seth R. J., Tighe P. et al. Quantitative analysis of IL-10 and IFN-γmRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. Pathology 195(2), 179–185 (2001). [DOI] [PubMed] [Google Scholar]
- Song S. H., Lee J. K., Lee N. W. et al. Interferon-γ (IFN-γ): A possible prognostic marker for clearance of high-risk human papillomavirus (HPV). Gynecol Oncol 108(3), 543–548 (2008). [DOI] [PubMed] [Google Scholar]
- Sanders Virginia M. Epigenetic regulation of Th1 and Th2 cell development. Brain Behav Immun 20(4), 317–324 (2006). [DOI] [PubMed] [Google Scholar]
- Northrop J. K., Thomas R. M., Wells A. D. et al. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. Immunology 177(2), 1062–1069 (2006). [DOI] [PubMed] [Google Scholar]