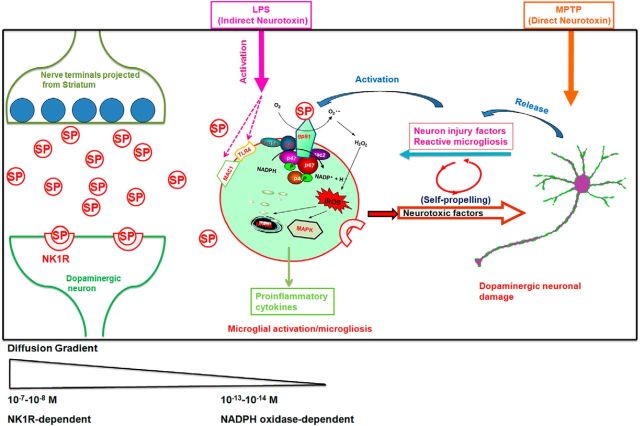
Figure 9.
Proposed model showing how SP exacerbates inflammatory responses and dopaminergic degeneration in the presence of LPS or MPTP in an NK1R-independent manner. Left, SP release from the nerve terminals projected from the striatonigral pathway. The synaptic concentrations of SP can reach submicromolar levels and act on NK1R to mediate acute synaptic transmission. Middle, Extrasynaptic actions of SP. Some of the released SP may escape the enzyme degradation and diffuse outside the synapses to activate the surrounding microglia. Our data show that subpicomolar concentrations of SP increase superoxide production and enhance iROS through the activation of microglial NOX2. The NK1R-independent activation of NOX2 by subpicomolar levels of SP could be a key pathway mediating the potentiated effects of LPS-induced activation of both MAPK and NF-κB and leading to an enhanced production of proinflammatory factors. Right, The mechanism by which the enhanced neuroinflammation produced by subpicomolar SP and neurotoxins causes dopaminergic neurodegeneration. Although LPS and MPTP exert neurotoxic effects through different mechanisms, the activation of microglia is directly involved in the action of LPS, whereas reactive microgliosis is indirectly involved in part of MPTP-induced neurotoxicity. The common pathway underlying the potentiating effect of subpicomolar SP on these two toxins is mediated by microglial NOX2. This schematic only shows the non–NK-1R-mediated neuroinflammatory and neurodegenerative effects of SP. In all likelihood, this neuropeptide can modulate microglial function via both NK-1R dependent and independent mechanisms, depending on the local SP concentrations.