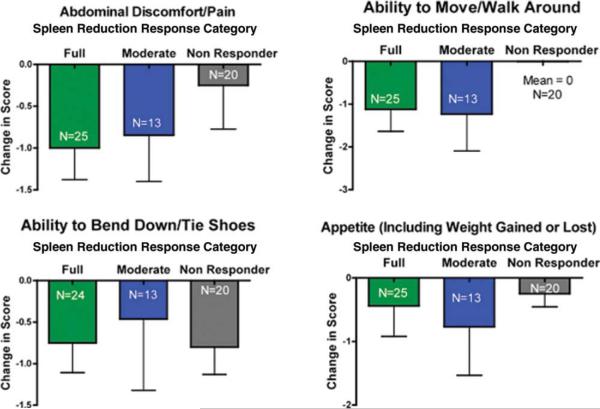
Figure 2.
Median change is shown in 4 splenomegaly-associated symptoms (abdominal discomfort/pain, ability to move/walk around, ability to bend/tie shoes, changed appetite) based on the level of spleen response after 6 cycles of therapy (ie, full, International Working Group for Myelofibrosis Research and Treatment [IWG-MRT] 50% reduction; partial, some splenic reduction but less than IWG-MRT threshold; and no response). Subjects with palpable baseline spleen and on-study responses after 6 cycles and who initiated therapy at doses of 10 mg twice daily, 15 mg twice daily, or 25 mg twice daily are included (n = 58).