Figure 1.
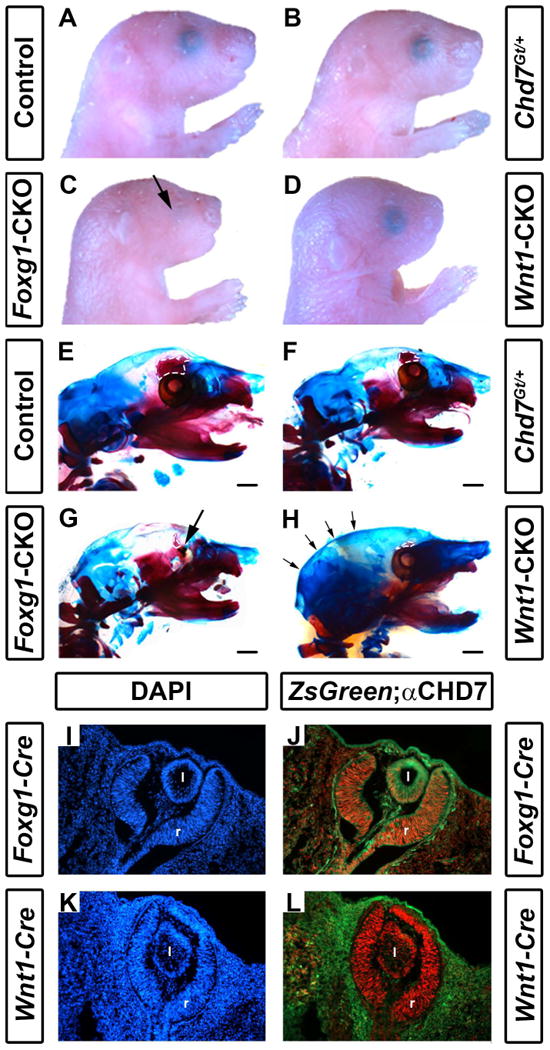
Conditional deletion of Chd7 results in ocular hypoplasia and cranial bone dysplasia. Brightfield images (A-D) and skeletal preparations (E-H) of postnatal day 1 control (Cre-negative, Chd7+/f or Chd7f/f) (A,E), Chd7Gt/+ (B,F), Foxg1-CKO (C,G) and Wnt1-CKO (D,H) pups. Chd7Gt/+ mice appear similar to controls, whereas both Foxg1-CKO (C) and Wnt1-CKO (D) mice have shortened snouts, and Foxg1-CKO mice have ocular hypoplasia (arrows in C and G). There are no abnormalities in ossification, overall morphology or size of major craniofacial bones in Chd7Gt/+ mice (B, F). In E-H, alizarin red and alcian blue staining reveal bone and cartilage, respectively. Ethmoid bones are hypoplastic in Chd7Gt/+ (F), Foxg1-CKO (G) and Wnt1-CKO (H) mice (white dashed lines in E-H). Wnt1-CKO mice (H) have a more rounded posterior cranium (indicated by arrows) than mice of other genotypes. Cre expression in Foxg1-Cre and Wnt1-Cre mice was detected using ZsGreen reporter mice and compared to CHD7 protein levels in the developing eye (I-L). Shown are transverse sections of Cre;ZsGreen positive E11.5 embryos stained with DAPI (labels cellular nuclei) or anti-CHD7 (red). Foxg1-Cre is active in CHD7-positive cells in the retina (r) and posterior lens (l) of the developing eye (J), whereas Wnt1-Cre is restricted to the surrounding periocular mesenchyme and is absent in the retina and lens proper (L). Scale bars in E-H are 1 mm.
