Abstract
Genetic variations in the wild-derived inbred mouse strains are more diverse than that of classical laboratory inbred mouse strains, including C57BL/6J (B6). The sleep/wake and monoamine properties of six wild-derived inbred mouse strains (PGN2, NJL, BLG2, KJR, MSM, HMI) were characterized and compared with those of B6 mice. All examined mice were nocturnal and had a polyphasic sleep pattern with a “main sleep period” identified during the light period. However, there were three sleep/wake phenotypic differences between the wild-derived mouse strains and B6 strain. First, the amount of sleep during the dark phase was comparable with that of B6 mice. However, the amount of sleep during the light phase was more varied among strains, in particular, NJL and HMI had significantly less sleep compared with that of B6 mice. Second, PGN2, NJL, BLG2, and KJR mice showed a “highly awake period” (in which the hourly total sleep time was <10%) immediately after the onset of the dark period, which was not seen in B6 mice. Third, relative to that of B6 mice, PGN2 and KJR mice showed longer duration of wakefulness episodes during the 12-h dark phase. Differences in whole brain noradrenaline, dopamine, and 5-hydroxy-tryptamine contents between the wild-derived mouse strains and B6 strain were also found. These identified phenotypes might be potentially under strong genetic control. Hence, wild-derived inbred mice could be useful for identifying the genetic factors underlying the regulation of sleep and wakefulness.
Keywords: dopamine, 5-hydroxy-tryptamine, noradrenaline, sleep, wakefulness
Introduction
Sleep is a complex brain function. However, several studies from humans and animals suggest that sleep is regulated by genetic means. Study of twins provides a unique method to investigate genetic influences in human sleep because monozygotic twins have higher similarity in sleep patterns compared with dizygotic twins [16, 26]. “Reverse genetics” (from gene to phenotype) with targeted gene transfer provides a powerful tool to evaluate genes that are already implicated in the regulation of sleep and wakefulness, such as interleukin-1 [22]. Indeed, interleukin-1 type-I receptor knockout mice have significantly less sleep during the dark phase compared with that of control mice [3]. “Forward genetics” (from phenotype to gene) with mutagenesis and quantitative trait loci (QTL) approaches can be used to scan in a genome-wide, unbiased fashion, which can be suitable for the discovery of “sleep genes”. Through linkage analyses and a positional cloning approach, Lin et al. demonstrated that familial narcolepsy in Dobermans and Labradors is caused by exon skipping mutations of the hypocretin (orexin) receptor 2 gene [14].
Inbred mouse strains have considerably surpassed 20 generations of inbreeding, and are homozygous in virtually all gene loci. Therefore, the strain differences in sleep and wakefulness phenotypes identified can be attributed to the differences in genotypes [24]. Franken et al. assessed genetic variations in the expression and regulation of sleep in classical laboratory inbred mice from six strains. They found striking strain differences in the amount of total sleep; onset and duration of the main sleep period; fragmentation of non-rapid eye movement sleep [4]; frequency of theta oscillations during rapid eye movement sleep [5]. Segregation of the latter trait was followed by extensive panels of inter- and back-crosses between BALB/cByJ with slow theta frequency and C57BL/6J (B6) with fast theta-peak frequency. The QTL approach revealed that the responsible gene linked tightly to theta-peak frequency was short-chain acyl-CoA dehydrogenase (Acads) on chromosome 5 [20].
Inbred mouse strains can be divided into two groups: classical laboratory and wild-derived. Classical laboratory mouse strains are used widely because of their advantages in availability, handling, breeding, and annotation database. However, they originate from relatively limited founder populations of fancy and other inbred mouse strains, so their genetic variation is limited [15]. The genomes of classical inbred strains are mosaic with mixed origin of four Mus musculus subspecies [25] with, on average, 68% being derived from Mus musculus domesticus [7]. Wild-derived inbred mouse strains are derived from mice captured in the wild at different locations that have been inbred to homozygosity through ≥20 generations of sibling mating [10]. Wild-derived mouse strains have much greater genetic variation than that of classical laboratory mice. Therefore, these mouse strains can be used as a powerful tool to examine the genetics underlying behavioral traits.
There are several reports on the characterization of sleep and wakefulness in classical laboratory mice but not in wild-derived inbred mouse strains. To provide a basis for identifying novel genetic factors involved in sleep and wakefulness, we evaluated sleep and wakefulness phenotypes in a panel of six inbred strains derived from wild mice. The wild-derived inbred mouse strains used were selected among four Mus musculus subspecies. That is, Mus musculus domesticus: PGN2/Ms (PGN2); Mus musculus musculus: NJL/Ms (NJL), BLG2/Ms (BLG2), KJR/Ms (KJR); Mus musculus castaneus: HMI/Ms (HMI); and Mus musculus molossinus: MSM/Ms (MSM). These mice are known to have diverse differences in behavioral characteristics such as spontaneous locomotor activity [9] and open-field behavior [21]. B6 (the most commonly used inbred laboratory mouse strain that belongs to Mus musculus domesticus) was used as a benchmark.
It is hypothesized that sleep is caused by reciprocal interactions between sleep and wake promoting brain regions, which produces a flip-flop switch. Monoaminergic neurons promote wakefulness by direct excitatory effects on the cerebral cortex and by inhibition of sleep-promoting neurons of the ventrolateral preoptic nucleus (VLPO) neurons in the hypothalamus. During sleep, the VLPO inhibits monoamine-mediated arousal regions [20]. Based on electrophysiological, neurochemical, neuropharmacological, and optogenetical approaches, it is accepted that noradrenaline (NA), 5-hydroxytryptamine (5-HT), and dopamine (DA) promote wakefulness and inhibit sleep [2, 17]. Therefore, brain contents of NA, 5-HT, and DA were also determined from seven inbred mouse strains to evaluate a potential relationship between brain monoamine contents and the observed sleep and wakefulness phenotypes.
Materials and Methods
Ethical approval of the study protocol
All animal experiments were conducted in accordance with the guidelines of The Guide for the Care and Use of Laboratory Animals (2011 version; National Institutes of Health, Bethesda, MD, USA). The study protocol was approved by the Animal Care and Use Committees at Hokkaido University (Hokkaido, Japan). All efforts were made to minimize the number of animals used and any pain and discomfort experienced by the subjects.
Animals
Fifty-four male adult mice from seven inbred strains (B6, PGN2, NJL, BLG2, KJR, MSM, HMI) were used [n = 8/strain; n = 6 for HMI]. Eight-week-old B6 mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Six wild-derived inbred mouse strains (PGN2, NJL, BLG2, KJR, MSM, HMI) aged 8–9 weeks were kindly provided by Dr. T. Shiroishi (National Institute of Genetics, Mishima, Japan). These mice were originally established as inbred strains from wild mice after 20 generations of full-sibling mating at the National Institute of Genetics.
The general information of mice, such as subspecies, origin, age (weeks), body length (cm), body weight (g), and brain weight (mg) at the end of sleep recording are summarized in Table 1. For more information regarding the behavioral characteristics of these mice, see Ref. 13. Mice were housed individually in polycarbonate cages and maintained in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care at an ambient temperature of 22 ± 2°C on a 12-h light-dark cycle (lights on at 7:00h). Standard laboratory chow (CE-2: CREA Japan, Tokyo, Japan) and distilled water were provided ad libitum.
Table 1. General information of seven inbred mouse strains.
| Strain | Subspecies | Origin | Weeks | Body length (cm) | Body weight (g) | Brain weight (mg) | |||
|---|---|---|---|---|---|---|---|---|---|
| B6 | Mus musculus domesticus | 12–13 | 9.3 ± 0.1 | 25.0 ± 0.2 | 503.3 ± 7.9 | ||||
| PGN2 | Mus musculus domesticus | Pegion, Canada | 11–12 | 8.9 ± 0.1 | * | 23.5 ± 0.3 | * | 437.0 ± 3.3 | * |
| NJL | Mus musculus musculus | Northern Jutland, Denmark | 10–13 | 8.4 ± 0.1 | * | 20.5 ± 0.3 | * | 392.5 ± 4.9 | * |
| BLG2 | Mus musculus musculus | Toshevo, Bulgaria | 13–14 | 8.0 ± 0.1 | * | 21.3 ± 0.5 | * | 371.6 ± 8.6 | * |
| KJR | Mus musculus musculus | Kojuri, Korea | 11–13 | 7.2 ± 0.1 | * | 13.4 ± 0.3 | * | 318.9 ± 5.7 | * |
| MSM | Mus musculus molossinus | Mishima, Japan | 12–17 | 7.1 ± 0.1 | * | 12.6 ± 0.3 | * | 312.0 ± 9.9 | * |
| HMI | Mus musculus castaneus | Hemei, Taiwan | 11–12 | 7.3 ± 0.1 | * | 16.1 ± 0.7 | * | 319.7 ± 5.4 | * |
Subspecies, origin, age (weeks), nose-to-anus length (cm), body weight (g), and brain weight (mg) at the end of sleep recording are summarized. Values are the means ± SE for 8 mice except for the HMI strain (n = 6). *P < 0.05 compared with the B6 strain.
Surgery and polygraphic recording
Mice were anesthetized with an intraperitoneal injection of a “drug cocktail” of ketamine (75 mg/kg) and medetomidine (1 mg/kg). They were then placed in a stereotaxic device, and chronically implanted with electroencephalography (EEG) and electromyography (EMG) electrodes for polysomnographic recording of sleep and wakefulness. Two stainless-steel screws (diameter, 1.0 mm) were implanted into the skull over the left cerebral hemisphere (frontal: 1.5 mm lateral to midline, 1.0 mm anterior to bregma; parietal: 1.5 mm lateral to midline, 1.0 mm anterior to lambda) according to Mouse Brain in Stereotaxic Coordinates [6]. A third screw was placed over the frontal bone and served as a ground electrode. EMG activity was monitored using stainless-steel Teflon-coated wires inserted bilaterally into the neck muscles. All electrodes were attached to a microconnector, and the entire assembly fixed to the skull with dental cement. Mice were injected with indomethacin (1 mg/kg, i.p.) immediately after surgery. All procedures were undertaken on a heating pad. Mice were allowed to recover for 10–14 days before being transferred to chambers where sleep recording was carried out.
After recovery, mice were housed individually in plastic cages (300 × 210 × 210 mm) in an insulated and soundproof recording chamber (illumination intensity, ≈100 lux). Mice were allowed ≥5 days to acclimatize to the recording environment. EEG, EMG, and spontaneous locomotor activity were recorded for 24 h, beginning at light onset [zeitgeber time (ZT) 0]. The recording cables were connected to a swivel contact so that movements of the animals were not restricted. Unihemispheric, frontal-parietal EEG and neck EMG signals were recorded on a BAS-8201 polygraph (Biotex, Kyoto, Japan). EEG and EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 16–128 Hz), digitized at a sampling rate of 128 Hz, and stored in 10-s epochs on a personal computer by using SleepSign ver.2 software (Kissei Comtec, Nagano, Japan). Spontaneous homecage locomotor activity was monitored via an infrared sensor (Biotex, Kyoto, Japan) mounted on the cage top. Locomotor activity was also amplified and then digitized at a sampling rate of 128 Hz and recorded by using SleepSign ver.2 software. After sleep recording, mice were weighed and sacrificed between 13:00 and 17:00 h. The brain was removed and stored at −80°C until the monoamine assay.
Determination of vigilance state
Vigilance states were classified offline automatically using SleepSign ver.2 based on EEG, EMG, and spontaneous locomotor activity in 10-s epochs into “sleep” and “wakefulness” using conventional criteria. Classified stages of sleep and wakefulness were confirmed visually and, if necessary, corrected.
Determination of monoamine contents
Whole-brain contents of NA, 5-HT and DA were assayed by modification of the high-performance liquid chromatography (HPLC) method of Refshauge et al. [18]. Brain tissues were homogenized in 0.2 N perchloric acid containing 1% sodium bisulfite and 1 mM ethylenediamine tetra-acetic acid. The homogenate was centrifuged at 5,000 × g for 10 min at room temperature. Aliquots of the resulting supernatant were treated with alumina at pH 8.5. After washing the alumina with distilled water, monoamines were eluted with 0.2 N acetic acid and assayed using an HPLC system with an electrochemical detector (Eicom, Kyoto, Japan).
Statistical analyses
Data are the mean ± SE. One-way analysis of variance followed by a Tukey-Kramer post hoc test was used to compare parameters of sleep and wakefulness among strains. P < 0.05 was considered significant.
Results
Body length, body weight and brain weight
General information on the seven inbred mouse strains are summarized in Table 1. In comparison with B6 mice, all wild-derived inbred mice were significantly shorter in body length as well as lighter in body weight and whole-brain weight.
Distribution of total sleep
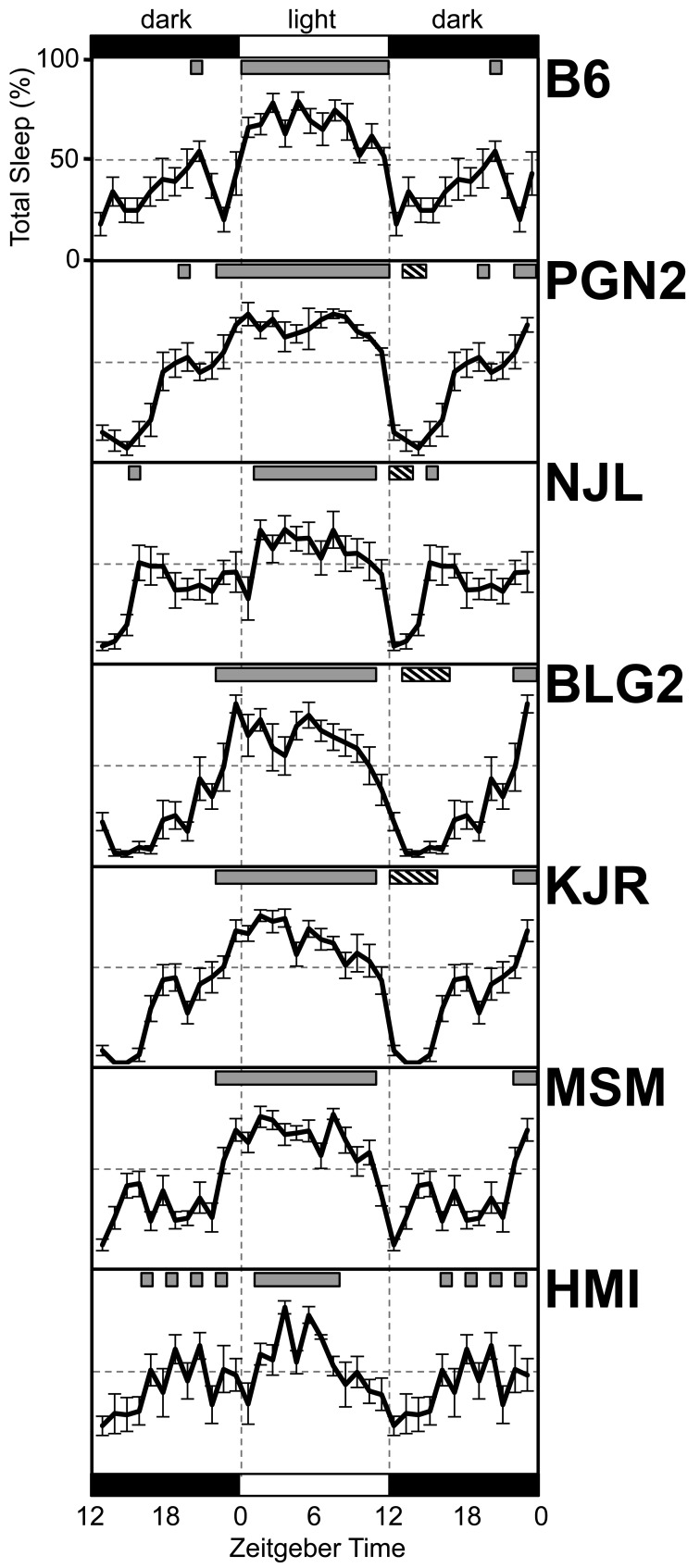
Analyses of 24-h recordings revealed that B6 mice exhibited a polyphasic sleep pattern with a peak hourly amount of total sleep (79.2%) at ZT 4 (where ZT 0 =light onset) and a minimum hourly amount of total sleep (18.1%) at ZT 12 (where ZT 12 = light offset) (Fig. 1). To understand the distribution pattern of sleep, we defined a block of “main sleep period” (MSP) as the longest block of hourly total sleep time exceeding 50%. According to this criterion, the MSP of B6 mice was synchronized completely with the entire 12-h light phase. Although primarily nocturnal, B6 mice also exhibited 54.2% of time asleep at ZT 20 of the dark phase.
Fig. 1.
Diurnal rhythm of total sleep in seven inbred mouse strains: B6, PGN2, NJL, BLG2, KJR, MSM, and HMI. The prevalence (%) of total sleep is plotted at 1-h intervals. Each data point represents the means ± SE for 8 mice except for the HMI strain (n = 6). The bars at the top and bottom represent the 12-h light (white) and 12-h dark (black) phases, respectively. The values for the 12-h dark phase are duplicated to illustrate the changes at the light–dark transition. Gray bars at the top of each figure represent the period of total sleep >50%. Hatched bars at the top of each figure represent periods of total sleep <10%.
In general, all examined wild-derived inbred mouse strains also exhibited a polyphasic sleep pattern with the MSP occurring during the 12-h light phase, a similar phenomenon to that seen in B6 mice. However, the distribution of the MSP varied considerably among wild-derived inbred mouse strains. The onset of the MSP of PGN2, BLG2, KJR, and MSM mice occurred at ZT22 of the dark phase, whereas that of NJL and HMI mice occurred at ZT1 of the light phase. The offset of the MSP of NJL, BLG2, KJR, and MSM mice occurred at ZT11, whereas that of HMI mice occurred at ZT8. The offset of the MSP of PGN2 mice was identical to that of B6 mice (ZT12). As a result, the duration of the MSP differed among wild-derived inbred strains, ranging from 7 h (HMI) to 14 h (PGN2). Similar to B6 mice, a large proportion of time spent asleep during the 12-h dark phase was observed for all examined wild-derived inbred strains, but their onset and offset differed greatly.
Total sleep
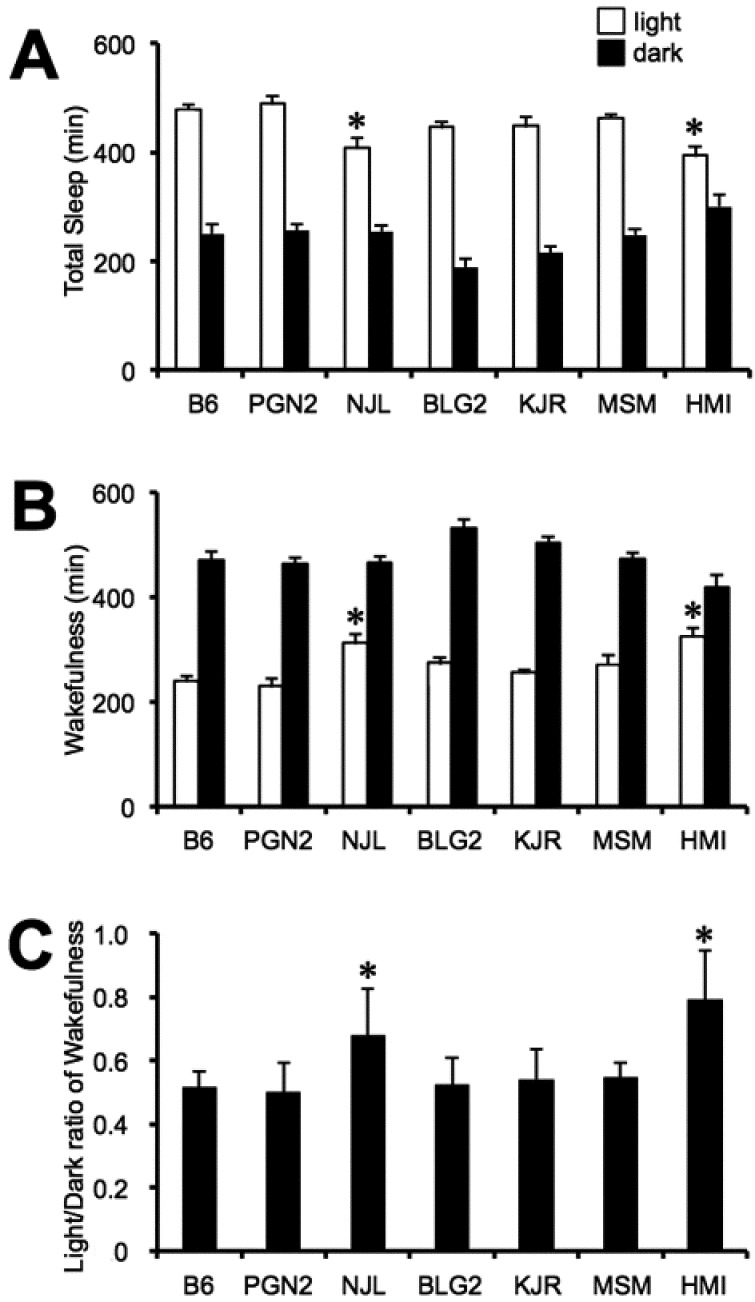
The total amount of sleep during the 12-h light and 12-h dark phases of B6 mice were 479.0 ± 7.8 min and 249.8 ± 17.2 min, respectively (Fig. 2A). The total sleep ratio between the 12-h light and 12-h dark phases (an indicator of diurnal variation in sleep) in B6 mice was calculated to be 1.98 ± 0.13. The total amount of sleep during the 12-h light phase (but not the 12-h dark phase) differed among the six mouse strains, ranging from 394.4 ± 16.1 min (HMI) to 489.5 ± 13.1 min (PGN2), and those of NJL and HMI mice were significantly less than that of B6 mice.
Fig. 2.
A: The amount of total sleep; B: The amount of wakefulness during the 12-h-light (open columns) and 12-h-dark (filled columns) phases. C: Light:dark ratio of wakefulness in seven inbred mouse strains. Values are the means ± SE for 8 mice except for the HMI strain (n = 6). *P < 0.05 compared with the B6 strain.
Wakefulness
Mice are considered to have a polyphasic sleep pattern if they have short sleep episodes lasting for several minutes at any time during a 24-h period. However, we noticed that some wild-derived mouse strains spent very little time asleep in the early dark phase (Fig. 1). Such a “highly awake period” (defined as an hourly total sleep time <10%) was observed in PGN2 (ZT13-14), NJL (ZT12-13), BLG2 (ZT13-16), and KJR (ZT12-15) mice.
The amount of wakefulness during the 12-h light and 12-h dark phases of B6 mice were 241.0 ± 7.8 min and 470.2 ± 17.2 min, respectively (Fig. 2B). The wakefulness ratio between the 12-h light and 12-h dark phases (an indicator of diurnal variation in wakefulness) in B6 mice was calculated to be 0.51 ± 0.02 (Fig. 2C). The amount of wakefulness during the 12-h light phase differed among the six mouse strains, ranging from 230.5 ± 13.1 min (PGN2) to 325.6 ± 16.1 min (HMI). The amount of wakefulness during the 12-h light phase of NJL and HMI mice (but not the other four strains) was significantly higher than that of B6 mice. In contrast, the amount of total wakefulness of the six wild-derived mouse strains during 12-h dark phase was not significantly different when compared with B6 mice. The wakefulness ratios between the 12-h light and 12-h dark phases of NJL (0.68 ± 0.05) and HMI (0.79 ± 0.02) mice were significantly greater than that of B6 mice.
Analyses of the structure of wakefulness
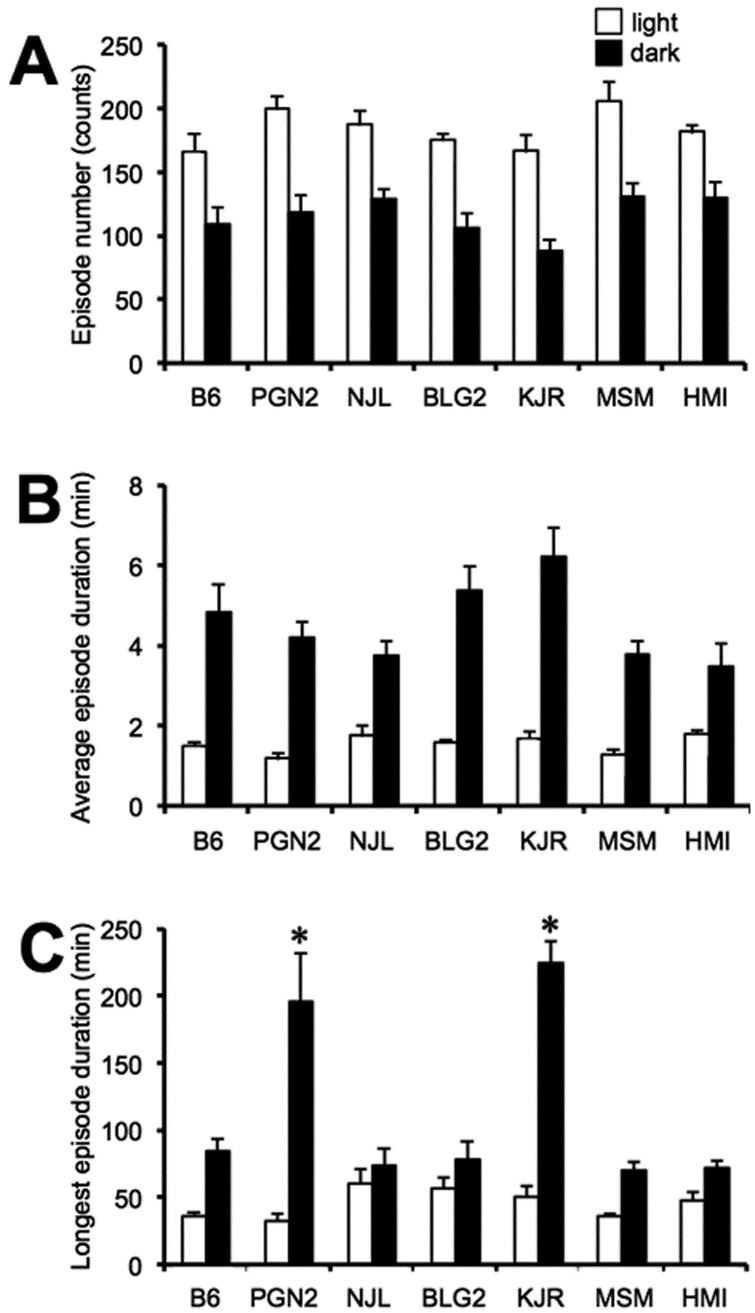
To evaluate the structure of wakefulness, the number and average duration of wakefulness periods were analyzed further. The number of wakefulness episodes during the 12-h light and 12-h dark phases of B6 mice was 166.3 ± 13.5 counts and 109.4 ± 13.1 counts, respectively (Fig. 3A). The average duration of wakefulness episodes during the 12-h light and 12-h dark phases of B6 mice was 1.5 ± 0.1 min and 4.8 ± 0.7 min, respectively (Fig. 3B). When the number and average duration of wakefulness periods in the six wild-derived mouse strains were compared with that of B6 mice, no significant differences were observed during 12-h light or 12-h dark phases.
Fig. 3.
A: Total number of episodes; B: Average duration of episode; C: Longest duration of episode of wakefulness during the 12-h light (open columns) and 12-h dark (filled columns) phases in seven inbred mouse strains. Values are the means ± SE for 8 mice except for the HMI strain (n = 6).*P < 0.05 compared with the B6 strain.
During determination of the vigilance state during the 12-h dark phase, we encountered exceptionally long bouts of wakefulness (>180 min) in KJR and PGN2 mice, whereas the longest wakefulness episode of B6 mice was 84.9 ± 9.3 min (Fig. 3C). When the longest bouts of wakefulness during the 12-h light phase of six wild-derived mouse strains were compared with that of B6 mice, no significant differences were observed.
Brain contents of NA, DA and 5-HT
Whole-brain contents of NA, DA, and 5-HT in the seven inbred mouse strains are summarized in Table 2. Whole-brain NA contents in PGN2, NJL, BLG2, and HMI mice were significantly higher than that of B6 mice. Whole-brain DA contents in BLG2, KJL, and MSM mice were significantly higher, whereas that of NJL mice significantly lower, than that of B6 mice. Whole-brain 5-HT contents in PGN2, NJL, BLG2, MSM, and HMI mice were significantly higher than that of B6 mice.
Table 2. Brain monoamine contents in seven inbred mouse strains.
| Strain | NA (ng/g) | DA (ng/g) | 5-HT (ng/g) | NA/DA ratio | 5-HT/DA ratio | 5-HT/NA ratio | |||||
| B6 | 891.7 ± 35.6 | 923.6 ± 48.8 | 888.2 ± 72.9 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | |||||
| PGN2 | 1,336.2 ± 52.9 | * | 800.6 ± 27.5 | 1,471.1 ± 71.6 | * | 1.7 ± 0.0 | * | 1.8 ± 0.1 | * | 1.1 ± 0.0 | |
| NJL | 1,188.9 ± 28.4 | * | 729.0 ± 31.0 | * | 1,366.5 ± 29.1 | * | 1.6 ± 0.1 | * | 1.9 ± 0.0 | * | 1.2 ± 0.0 |
| BLG2 | 1,168.6 ± 59.0 | * | 1,317.1 ± 45.3 | * | 1,411.1 ± 33.6 | * | 0.9 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.1 | ||
| KJR | 923.3 ± 101.2 | 1,395.7 ± 34.4 | * | 1,093.6 ± 36.0 | 0.7 ± 0.1 | * | 0.8 ± 0.0 | 1.2 ± 0.2 | |||
| MSM | 904.0 ± 25.1 | 1,280.3 ± 41.3 | * | 1,191.8 ± 25.8 | * | 0.7 ± 0.0 | * | 0.9 ± 0.0 | 1.3 ± 0.1 | ||
| HMI | 1,993.7 ± 50.1 | * | 976.8 ± 17.2 | 2,225.1 ± 41.8 | * | 2.0 ± 0.0 | * | 2.3 ± 0.1 | * | 1.1 ± 0.1 | |
Whole-brain contents of NA, DA, and 5-HT (ng/ g tissue) of seven inbred mouse strains are summarized. Based on these data, NA/DA, 5-HT/DA, 5-HT/NA ratios were calculated. Values are the means ± SE for 4–6 mice. *P < 0.05 compared with the B6 strain.
NA/DA, 5-HT/DA, and 5-HT/NA ratios were calculated to compare the whole-brain patterns of monoamine distribution of wild-derived inbred mice with those of B6 mice. There were no significant differences in the 5-HT/NA ratio among inbred strains. However, PGN2, NJL, and HMI mice showed higher NA/DA and 5-HT/DA ratios than those of B6 mice, whereas KJR and MSM mice showed a lower NA/DA ratio.
Discussion
In this study, we evaluated sleep and wakefulness in a panel of six wild-derived inbred mouse strains among four Mus musculus subspecies in addition to the commonly used classical laboratory inbred B6 mice. Although we were not able to observe any sleep and wakefulness phepotypes that characterize a particular Mus musculus subspecies, we were able to observe several unique phenotypes that characterize particular inbred mice strains. B6 mice exhibited a polyphasic sleep pattern with a MSP that was synchronized completely with the entire 12-h light phase. Although primarily nocturnal, B6 mice also exhibited a large proportion of time asleep at ZT 20 of the dark phase. These observations are quite consistent with previous reports [4, 23]. Other major classical laboratory mouse strains (including DBA/2J and 129/Ola mice) also have a MSP identical to the 12-h light phase [4]. Hence, we speculate that mice with sleep and wakefulness patterns adjusted to the light-dark cycle may be selected preferably during the breeding of laboratory inbred mouse strains.
All examined wild-derived inbred mouse strains were also nocturnal, and the amount of sleep during the dark phase was comparable with that of B6 mice. However, the amount of sleep during the light phase was more varied among strains. In particular, NJL and HMI mice had significantly less sleep compared with B6 mice. All the wild-derived inbred mice examined in our study had a polyphasic sleep pattern. Also, the onsets and offsets of the MSP were varied among strains similar to studies focusing on classical laboratory inbred mouse strains [4]. The onsets of the MSP of PGN2, NJR, MSM and BLG2 mice occurred before light onset at ZT22, whereas that of NJL and HMI mice occurred after light onset at ZT1. The offset of the MSP of NJL, BLG2, KJR, and MSM mice occurred at ZT11, whereas that of HMI mice occurred at ZT8. It is well known that light exerts a direct effect on sleep and wakefulness in nocturnal and diurnal animals. For example, a light pulse during the dark phase suppresses locomotor activity and promotes sleep in nocturnal animals [1]. Therefore, the response to light may have a variation among mice strains that helps to determine the timing of the onset or offset of sleep.
The amount of wakefulness during the dark phase was comparable between the B6 mice and wild-derived mice strains examined, but that of the light phase were more varied among mouse strains. In particular, NJL and HMI mice had significantly more wakefulness compared with B6 mice. When the wakefulness ratio between 12-h light and 12-h dark phases was calculated, HMI and NJL mice had significantly higher values than B6 mice, suggesting the low diurnal amplitude of the wakefulness rhythm. In support of our HMI data, the low diurnal amplitude of locomotor activity rhythm has also been reported in CAST/Ei mice (who also belong to the same Mus musculus castaneus subspecies) [13]. In the present study, HMI mice also had a short MSP that terminated 4 h before dark onset (ZT8). That is, the offset of the MSP is the onset of the active period, so these mice have an early onset of activity rhythm. In support of this idea, CAST/Ei mice also have an “early runner phenotype” because the onset of their wheel-running activity is 2.7-h before the onset of the dark period [12]. When sleep/wakefulness profiles were assessed, CAST/Ei mice exhibited early daily awakening and spent more time awake than B6 mice in the last 4 h of the light phase [11]. Therefore, early onset of activity rhythm as well as a low diurnal amplitude of the wakefulness rhythm are common features in these mice strains. Sleep and wakefulness phases vary considerably in patients with advanced or delayed-sleep phase syndrome, and these disorders are thought to reflect misalignment between endogenous circadian rhythms and external cues [19]. Hence, HMI and CAST/Ei mice will be useful models of sleep disorders of circadian rhythms in humans.
We also observed a highly awake period in which the hourly total sleep time was <10% during the early light period in PGN2, NJL, BLG2, and KJR mice. In particular, the duration of this period reached 4 h in BLG2 and KJR mice. According to the structural analyses of wakefulness in PGN2, NJL, BLG2, and KJR mice, the overall number of episodes as well as average duration of an episode of wakefulness was comparable with those of B6 mice. However, when the longest waking episode of the day was compared among inbred mouse strains, those of KJR and PGN2 mice were significantly elongated in comparison with that of B6 mice. Therefore, the highly awake period of KJR and PGN2 mice comprised exceptionally long waking episodes, and was distinguishable with that of NJL and BLG2 mice. We did not video-monitor mice behavior in conjunction with sleep recording so characterizing the specific behavior related to the highly awake period was difficult. However, considering the fact that, in general, classical laboratory mice consume large amounts of food at the early dark phase [8], the highly awake period may be related to feeding and food exploration.
The distribution of sleep and wakefulness through a 24-h period is dependent upon endogenous mechanisms. Groups of neurons projecting to the cerebral cortex and thalamus, which transmit the arousal signals of neurotransmitters such as NA, DA, 5-HT, acetylcholine, histamine, and orexin, receive excitatory inputs originating from the brainstem, hypothalamus, and basal forebrain [8]. Enhancement of neural activity in the noradrenergic locus coeruleus by an optogenetic approach demonstrated directly the causal role for the locus coeruleus in promoting and maintaining arousal [2]. Based on electrophysiological, neurochemical, and neuropharmacological approaches, it is accepted that 5-HT and DA promote wakefulness and inhibit sleep [17]. Therefore, we measured NA, DA, and 5-HT contents in the whole brain, which reflects approximately the density of the noradrenergic, dopaminergic, and serotonergic projections in the brain, respectively. According to the monoamine contents in the brain, inbred mouse strains were categorized as “high NA group” (PGN2, NJL, BLG2, and MSM); “high DA group” (BLG2, KJR, and MSM); and “high 5-HT group” (PGN2, NJL, BLG2, MSM, and HMI). However, there was no obvious relationship between whole-brain contents of NA, DA, and 5-HT in relation to sleep and wakefulness phenotypes. Next, to examine the relative contribution of monoaminergic transmission to sleep and wakefulness, NA/DA, 5-HT/DA, and 5-HT/NA ratios were calculated. The 5-HT/NA ratio was relatively constant among inbred strains, even though their brain contents varied considerably. According to the calculated monoamine ratios, inbred mouse strains were categorized as “high NA/DA, high 5-HT/DA group” (PGN2, NJL, and HMI); “low NA/DA group” (KJR and MSM); and “B6 group” (B6 and BLG2). These classifications provided a possible relationship to sleep and wakefulness as a function of mouse strain. For example, mice categorized as the high NA/DA, high 5-HT/DA group (NJL and HMI) were found to possess a low diurnal amplitude of wakefulness rhythm. The B6 group (B6 and BLG2) was found to possess similarities in the daily amount of sleep and wakefulness as well as structure of wakefulness. Observed monoaminergic properties may help in understanding the biochemical basis underlying phenotypic differences of sleep and wakefulness.
In summary, several unique sleep and wakefulness phenotypes were observed. These phenotypes might be potentially under strong genetic control. Hence, wild-derived inbred mice could be useful for identifying the genetic factors underlying the regulation of sleep and wakefulness.
Acknowledgments
We thank Dr. Tsuyoshi Koide (Mouse Genomics Laboratory, National Institute of Genetics) for valuable discussions. This work was supported in part by Grants-in-aid for scientific research from the Japan Society for the Promotion of Science (grant number 22500830) and the Akiyama Foundation.
References
- 1.Alföldi P., Franken P., Tobler I., Borbély A.A.1991. Short light-dark cycles influence sleep stages and EEG power spectra in the rat. Behav. Brain Res. 43: 125–131. doi: 10.1016/S0166-4328(05)80062-2 [DOI] [PubMed] [Google Scholar]
- 2.Carter M.E., Yizhar O., Chikahisa S., Nguyen H., Adamantidis A., Nishino S., Deisseroth K., de Lecea L.2010. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13: 1526–1533. doi: 10.1038/nn.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang J., Wang Y., Krueger J.M.1998. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am. J. Physiol. 274: R655–R660. [DOI] [PubMed] [Google Scholar]
- 4.Franken P., Malafosse A., Tafti M.1999. Genetic determinants of sleep regulation in inbred mice. Sleep 22: 155–169. [PubMed] [Google Scholar]
- 5.Franken P., Malafosse A., Tafti M.1998. Genetic variation in EEG activity during sleep in inbred mice. Am. J. Physiol. 275: R1127–R1137. [DOI] [PubMed] [Google Scholar]
- 6.Franklin K.G.B., Paxinos G.1997. The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA [Google Scholar]
- 7.Frazer K.A., Eskin E., Kang H.M., Bogue M.A., Hinds D.A., Beilharz E.J., Gupta R.V., Montgomery J., Morenzoni M.M., Nilsen G.B., Pethiyagoda C.L., Stuve L.L., Johnson F.M., Daly M.J., Wade C.M., Cox D.R.2007. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448: 1050–1053. doi: 10.1038/nature06067 [DOI] [PubMed] [Google Scholar]
- 8.Fuller P.M., Gooley J.J., Saper C.B.2006. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms 21: 482–493. doi: 10.1177/0748730406294627 [DOI] [PubMed] [Google Scholar]
- 9.Furuse T., Takano-Shimizu T., Moriwaki K., Shiroishi T., Koide T.2002. QTL analyses of spontaneous activity by using mouse strains from Mishima battery. Mamm. Genome 13: 411–415. doi: 10.1007/s00335-002-2168-5 [DOI] [PubMed] [Google Scholar]
- 10.Guénet J.L., Bonhomme F.2003. Wild mice: an ever-increasing contribution to a popular mammalian model. Trends Genet. 19: 24–31. doi: 10.1016/S0168-9525(02)00007-0 [DOI] [PubMed] [Google Scholar]
- 11.Jiang P., Franklin K.M., Duncan M.J., O’Hara B.F., Wisor J.P.2012. Distinct phase relationships between suprachiasmatic molecular rhythms, cerebral cortex molecular rhythms, and behavioral rhythms in early runner (CAST/EiJ) and nocturnal (C57BL/6J) mice. Sleep 35: 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P., Striz M., Wisor J.P., O’Hara B.F.2011. Behavioral and genetic dissection of a mouse model for advanced sleep phase syndrome. Sleep 34: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koide T., Moriwaki K., Ikeda K., Niki H., Shiroishi T.2000. Multi-phenotype behavioral characterization of inbred strains derived from wild stocks of Mus musculus. Mamm. Genome. 11: 664–670. doi: 10.1007/s003350010129 [DOI] [PubMed] [Google Scholar]
- 14.Lin L., Faraco J., Li R., Kadotani H., Rogers W., Lin X., Qiu X., de Jong P.J., Nishino S., Mignot E.1999. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98: 365–376. doi: 10.1016/S0092-8674(00)81965-0 [DOI] [PubMed] [Google Scholar]
- 15.Lindblad-Toh K., Winchester E., Daly M.J., Wang D.G., Hirschhorn J.N., Laviolette J.P., Ardlie K., Reich D.E., Robinson E., Sklar P., Shah N., Thomas D., Fan J.B., Gingeras T., Warrington J., Patil N., Hudson T.J., Lander E.S.2000. Large-scale discovery and genotyping of single-nucleotide polymorphisms in the mouse. Nat. Genet. 24: 381–386. doi: 10.1038/74215 [DOI] [PubMed] [Google Scholar]
- 16.Linkowski P., Kerkhofs M., Hauspie R., Susanne C., Mendlewicz J.1989. EEG sleep patterns in man: a twin study. Electroencephalogr. Clin. Neurophysiol. 73: 279–284. doi: 10.1016/0013-4694(89)90106-5 [DOI] [PubMed] [Google Scholar]
- 17.Monti J.M., Jantos H.2008. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog. Brain Res. 172: 625–646. doi: 10.1016/S0079-6123(08)00929-1 [DOI] [PubMed] [Google Scholar]
- 18.Refshauge C., Kissinger P.T., Dreiling R., Blank L., Freeman R., Adams R.N.1974. New high performance liquid chromatographic analysis of brain catecholamines. Life Sci. 14: 311–322. doi: 10.1016/0024-3205(74)90061-7 [DOI] [PubMed] [Google Scholar]
- 19.Sack R.L., Auckley D., Auger R.R., Carskadon M.A., Wright K.P., Jr, Vitiello M.V., Zhdanova I.V., American Academy of Sleep Medicine2007. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 30: 1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tafti M., Petit B., Chollet D., Neidhart E., de Bilbao F., Kiss J.Z., Wood P.A., Franken P.2003. Deficiency in short-chain fatty acid beta-oxidation affects theta oscillations during sleep. Nat. Genet. 34: 320–325. doi: 10.1038/ng1174 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi A., Kato K., Makino J., Shiroishi T., Koide T.2006. Multivariate analysis of temporal descriptions of open-field behavior in wild-derived mouse strains. Behav. Genet. 36: 763–774. doi: 10.1007/s10519-005-9038-3 [DOI] [PubMed] [Google Scholar]
- 22.Terao A., Matsumura H., Saito M.1998. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J. Neurosci. 18: 6599–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terao A., Steininger T.L., Hyder K., Apte-Deshpande A., Ding J., Rishipathak D., Davis R.W., Heller H.C., Kilduff T.S.2003. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience 116: 187–200. doi: 10.1016/S0306-4522(02)00695-4 [DOI] [PubMed] [Google Scholar]
- 24.Valatx J.L., Bugat R., Jouvet M.1972. Genetic studies of sleep in mice. Nature 238: 226–227. doi: 10.1038/238226a0 [DOI] [PubMed] [Google Scholar]
- 25.Wade C.M., Kulbokas E.J., 3rd, Kirby A.W., Zody M.C., Mullikin J.C., Lander E.S., Lindblad-Toh K., Daly M.J.2002. The mosaic structure of variation in the laboratory mouse genome. Nature 420: 574–578. doi: 10.1038/nature01252 [DOI] [PubMed] [Google Scholar]
- 26.Young J.P., Lader M.H., Fenton G.W.1972. A twin study of the genetic influences on the electroencephalogram. J. Med. Genet. 9: 13–16. doi: 10.1136/jmg.9.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]