Abstract
The adult central nervous system has only a limited capacity for axonal regeneration. In this study, fibroblast growth factor-2 (FGF-2) was injected once into the spinal cord tissue around the injury site immediately after complete spinal cord transection in rats. This treatment markedly improved the locomotor function of the animals. Histological analysis demonstrated that tissue composed of FGF-2-induced fibronectin-positive cells (FIFs) had infiltrated the injury site and filled large cystic cavities, into which numerous axons with growth-associated protein-43 immunoreactivity penetrated. The FIFs could also be cultured from the intact spinal cord tissue, demonstrating that they were resident in the non-injured spinal cord. They had a spindle-shaped morphology and enhanced expression of mRNAs of N-cadherin and neurotrophic factors, suggesting the beneficial properties of the FIFs for axonal regeneration. Thus, these results argue for the continual use of autologous transplantation as a novel and promising cell therapy for the treatment of spinal cord injury.
Key words: : axonal regeneration, cell transplantation, growth factors, locomotor function, spinal cord injury
Introduction
Impairment of voluntary motor function after a spinal cord injury (SCI) frequently disrupts the descending motor pathways at the injury site.1 Such damage causes a permanent loss of function and paralysis, because the regenerative response is critically limited. The failure of axonal regeneration after injury to the central nervous system (CNS) of mammals is thought to be due to a number of factors, including the following: (1) the presence of a tissue environment at the lesion site that inhibits axonal growth, including large cystic cavities2 and astroglial scars3,4); (2) the presence of inhibitory molecules in myelin such as Nogo, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein, which may interact with neuronal receptors to induce intracellular signaling to suppress axon growth5,6; (3) failure of the formation of functional synaptic constructs7,8; and (4) a lack of adequate neurotrophic support, which may lead to retrograde death and/or inhibit the activation of genes advantageous for axonal regeneration of neurons whose axons were injured.9–12
Fibroblast growth factors (FGFs) have been implicated in numerous cellular processes, including proliferation, migration, differentiation, and survival. In particular, FGF-2 stimulates the growth of blood vessels,13 which is crucial for forming nervous system tissue,14 and enhances neurogenesis.15–17 Further, FGF-2 facilitates neuronal survival and neuritegenesis,18 and positively influences properties of oligodendrocytes.19 These varied effects of FGF-2 on the CNS may be useful for tissue sparing and functional recovery following moderate or severe contusive spinal cord injury (SCI).20,21
In this study, we performed complete transection of the spinal cord, an injury much more severe than a contusion,22 and spontaneous recovery is unexpected. FGF-2 was directly injected into the tissues around the injury site, since heparan sulfate proteoglycans present on cell surfaces and/or the extracellular matrix are reported to activate, stabilize, and preserve FGF-2.23.24 This administration improved locomotor function, as expected; however, FGF-2-induced fibronectin‐positive cells (FIFs) were suspected to be involved in the restoration, because the large cystic cavities around the lesion site were filled with these cells. We confirmed this possibility by transplantation of the cultured FIFs into the lesion sites of other cord-transected rats. The most effective FIFs seemed to be spinal cord–resident mesenchymal stem cells (MSCs), which were critically different from bone marrow-derived MSCs, or meninges-derived fibronectin-positive cells (MDFs).
Methods
FGF-2
Escherichia coli cells producing recombinant mouse 18-kDa FGF-2, in which Cys78 and Cys96 had been replaced with Ser residues to improve stability, were a generous gift from Drs. Yoshitake Y. and Nishikawa K. of Kanazawa Medical University, Ishikawa Prefecture, Japan. Recombinant mouse FGF-2 was purified according to the method described previously.25 Human recombinant FGF-2 was a gift from Kaken-Pharmaceutical, Co., Ltd., Kyoto, Japan. Further, we used a recombinant mouse FGF-2 purchased from R&D Systems, Inc. (Minneapolis, MN). The biological activity of FGF-2 to induce the proliferation of fibroblasts cultured from injured spinal cord tissue was substantial (data not shown).
SCI followed by administration of FGF-2
The animals were handled in accordance with the Guidelines of Experimental Animal Care issued by the Office of the Prime Minister of Japan. Female Wistar rats (7 weeks of age, weighing 120–140 g; SLC, Hamamatsu, Japan) were used in this study. The rats were anesthetized with sodium pentobarbital (40-mg/kg body weight), and the spinal cord was completely transected with microsurgical scissors after laminectomy at the level of the 10th thoracic vertebra. The distal stump was carefully lifted up to confirm complete transection. We also ensured that the distal stump and the proximal stump were in contact when the former was returned to its original position. After arrest of hemorrhage, 5 μL of vehicle (phosphate‐buffered saline, PBS; vehicle-treated group), or FGF-2 dissolved in PBS (1 μg/μL; FGF-2-treated group) was injected in multisite fashion into the cord tissue 1.5-mm rostral and 1.5-mm caudal from the epicenter of the lesion via a glass microcapillary tube (GD-1; Narishige, Tokyo, Japan) attached to a microinjector (PB-7; Narishige). After administration of FGF-2 or vehicle, the muscle and skin were sutured closed. The rats were then placed in standard cages and given free access to food and water. Manual bladder evacuation was performed twice a day until bladder function recovered.
Assessment of locomotor function
BBB locomotor scale
Hindlimb locomotor function was assessed by use of the Basso, Beattie, and Bresnahan (BBB) locomotor scale in an open field, as described earlier.26 Evaluation was performed 1 day after injury; it was subsequently done once a week by observers blinded to experimental treatment and was continued up to 6 weeks after injury.
Inclined plane test
Functional recovery of locomotion activity was also evaluated in terms of the ability of the treated rats to maintain their body position on an inclined board. The angle of incline was increased incrementally by 5 degrees. The maximum angle at which the animal could still maintain its position on the board for 10 sec was recorded for each animal. This test was performed 1, 21, and 42 days after injury.
Retrograde axonal tracing
Rats were anesthetized with sodium pentobarbital 6 weeks after cord injury, and laminectomy was performed at the level of the first lumbar vertebra. The animals were then given stereotaxic multisite injections of 5 μL of 4% fluorogold (FG; Fluorochrome, Denver, CO) 5-mm caudal to the lesion site with a glass pipette attached to a microinjector. As the FG was expected to be retrogradely transported within axons into the nuclei from which regenerating axons originate, the rats were killed 3 days after injection and processed for tissue samples as described below. Observations were focused on the sensory motor cortex and the red nucleus. The fluorescence signal of the FG was histochemically monitored with a microscope (Axiovert S 100; Zeiss, Jena, Germany) or quantitatively evaluated with a fluorophotometer. For quantitative measurements, the cerebral sensory motor cortices were sonicated in PBS (5% w/v), and the homogenates were centrifuged at 3×104 g for 20 min. The fluorescence intensity of the supernatant fluids was measured at 418 nm with excitation at 331 nm.
Tissue preparation for histological analysis
The animals were deeply anesthetized by an intraperitoneal (IP) injection of sodium pentobarbital, and then cardio-perfused with 4% paraformaldehyde (PFA) in 0.1-M phosphate buffer (pH 7.4). The spinal cord tissues including the lesion site were dissected out and post-fixed in the same fixative overnight at 4°C. The tissues were then soaked in cold PBS containing 20% sucrose for 15 h, and frozen in embedding compound (Sakura Finetek, Tokyo, Japan). The frozen sections (20 or 25-μm thickness) were prepared with a cryostat (model CM 1800; Leica, Nussloch, Germany), placed on adhesive-coated slides (Matsunami, Osaka, Japan), and histologically analyzed under a light microscope (Axiovert S 100; Zeiss) after hematoxylin and eosin staining.
Immunohistochemical analysis
For immunostaining, spinal cord sections were fixed with 4% PFA solution for 10 min at 37°C and subsequently boiled in Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) by autoclaving for 15 min at 121°C. The sections were then rinsed for 30 min at 37°C in 0.1-M Tris-HCl buffer (pH 7.4) containing 0.3% (v/v) Triton X 100. After washing with PBS, the sections were blocked for 30 min at room temperature in PBS containing 2% Block Ace, and then reacted with the diluted primary antibodies for appropriate times at 4°C. After another wash with PBS, the sections were incubated with the appropriate fluorescence-conjugated secondary antibodies for 3 h at room temperature. The following primary antibodies were used in this study: polyclonal rabbit anti-glial-fibrillary acidic protein (GFAP; 1:1000; Dakocytomation, Glostrup, Denmark); rabbit anti-fibronectin (1:1000; LSL, Tokyo, Japan); and monoclonal mouse anti-GAP-43 (1:1000; Millipore, Temecula, CA). These secondary antibodies were used: Alexa 488-conjugated goat anti-mouse immunoglobulin G (IgG), and Alexa 546-conjugated goat anti-rabbit IgG (1:1000; Invitrogen, Carlsbad, CA). The slides were washed with PBS and coverslipped with PermaFluor Aqueous Mounting Medium (Thermoshandon, Waltham, CA). The images were observed with a confocal laser microscope (LSM 510; Zeiss).
Cell labeling with 5-bromo-2′-deoxyuridine (BrdU)
Five groups of rats (n=3 per group) were used for a BrdU cell-labeling assay. For BrdU labeling, 50-μg/g body weight of BrdU (Sigma Aldrich, St. Louis, MO) in sterile PBS was injected IP into the rats. Immediately after that, the animals were sham-operated (sham group), received the vehicle injection without SCI (vehicle group), received FGF-2 injection without SCI (FGF-2 group), received vehicle injection with SCI (SCI/vehicle group), or received FGF-2 injection with SCI (SCI/FGF-2 group). The animals were cardio-perfused with 4% PFA 2 days after surgery. Subsequent procedures were performed as described in the previous sections. We prepared coronal sections 20-μm thick at the position 1-mm caudal to the injury site. For detection of the BrdU-labeled cells, the tissue sections were treated sequentially with 1-N HCl for 30 min at 60°C, and 0.25% (w/v) trypsin for 30 sec at 37°C in PBS to unmask the BrdU molecules. Then the sections were incubated with anti-BrdU mouse antibody (1:500; Sigma Aldrich), anti-BrdU sheep antibody (1:500; Abcam, Cambridge, U.K.), and/or the diluted primary antibodies for the various cell markers. The sections were subsequently reacted with fluorescence-conjugated secondary antibodies. The following primary antibodies were used in this study: polyclonal rabbit anti-GFAP (1:1000; Dakocytomation), rabbit anti-fibronectin (1:1000; LSL), mouse anti-OX-42 (1:200; Serotec, Oxford, U.K.), monoclonal mouse anti-βIII-tubulin (1:1000; Promega, Madison, WI), and polyclonal mouse anti-NG2 (1:500; Millipore). The secondary antibodies used were: Alexa 488- or Alexa 546-conjugated goat anti-mouse IgG, Alexa 488-conjugated goat anti-rabbit IgG, and Alexa 546-conjugated goat anti-sheep IgG (all antibodies were from Invitrogen).
We counted a cell as being BrdU-positive when the cell showed BrdU staining with a diameter of more than 1 μm in its nucleus. BrdU-positive cells were counted in seven arbitrarily selected areas 5.3×10–2 mm2 within each section. The value was expressed as the fold increase of that over the sham-operated group. In the double-immunostained sections, the percentages of BrdU-positive cells co-expressing each cell marker of the total BrdU-positive cells were calculated.
Primary cultures
FIFs
The animals were processed as described in the section above on animal surgery. Briefly, after transection of the spinal cord, FGF-2 was administered into the tissue around the injury site, and the rats were fed for 2 days. Then the spinal cord tissue including the lesion site was dissected out, and the meninges were carefully removed and discarded. The rostral and caudal stumps of the injury site were separately collected, and each was cut further into dorsal and ventral parts. These four pieces were explanted separately on 6-cm dishes coated with collagen (Cell Matrix Type IV; Nitta Gelatin, Osaka, Japan), with the central canal turned toward the dish surface, and cultured in 1 mL of Dulbecco's modified Eagle's medium (DMEM), supplemented with FGF-2 (10 ng/mL) and 10% fetal bovine serum (FBS) (HyClone, South Logan, UT). After sufficient cell migration from the explants had occurred, the explants were briefly removed, and fresh medium was added. The cells vigorously grew, and showed a fibroblast-like morphology with expression of fibronectin. Therefore, we termed them FIFs. The FIFs were cultured from individual animals, and used separately for the following experiments.
Meninges-derived fibronectin-positive cells (MDFs)
The animals were processed as described above for FIFs, except FGF-2 was not administered. Briefly, 2 days after SCI, the meninges were collected separately from the spinal cord, cut into fragments, and cultured on collagen-coated culture dishes in FGF-2-free DMEM supplemented with 10% FBS. The cells that had sufficiently migrated and grown were detached from the culture vessel by trypsin treatment, and cultured in medium containing both FGF-2 and FBS at least for another 3 days. These cells also grew vigorously and showed a fibroblast-like morphology with expression of fibronectin, as did the FIFs, and we termed them meninges-derived fibronectin-positive cells (MDFs). MDFs from individual animals were cultured, grown separately, and used for the experiments, as was the case for the FIFs.
Cortical neurons
Cortical neurons were cultured from the cerebral cortex of mouse embryos as described previously.27 Briefly, the cerebral cortex was separated from the cerebrum of 17-day-old mouse embryos and incubated for 15 min at 37°C in PBS containing 0.25% trypsin (Becton Dickinson and Company, Sparks, MD) and 0.5% D glucose (Wako Pure Chemical Industries, Osaka, Japan). The dissociated neurons were suspended and cultured in medium containing FBS (1%; HyClone), transferrin (5 μg/mL; Sigma Aldrich), insulin (5 μg/mL; Sigma Aldrich), progesterone (2 pM, Sigma Aldrich), and bovine serum albumin (0.5%; Nacalai Tesque, Kyoto, Japan).
Influence of FIFs or MDFs on neurite outgrowth from cortical neurons
FIFs or MDFs from three animals were grown separately to confluence on poly-L-lysine-coated coverslips (13-mm diameter; Nalge Nunc International, Naperville, IL) and placed in 24 well plates. About 50,000 cortical neurons per well were seeded onto a monolayer of FIFs or MDFs and cultured for 2 days. Thereafter the cultures were stained for βIII-tubulin antigen, a specific marker of neurons. One hundred neurons were randomly picked per well from each of the three cultures, and the percentage of the neurons with neurites longer than 150 μm was calculated.
Next, neurite outgrowth-promoting activity secreted into the medium by FIFs or MDFs was examined. FIFs or MDFs from each of three animals were grown to confluence, maintained for 2 days in the medium for neuronal cultures, and the conditioned media (CM) was collected. Cortical neurons (about 50,000) were seeded onto poly-L-lysine-coated 13-mm coverslips that had been placed in 24 well plates, and cultured for 2 days in the CM diluted two times with fresh medium. The cells were then fixed with 4% PFA solution, and the neurites were stained as described above. One hundred neurons were arbitrarily selected per well from each of the three cultures, and the percentage of neurons with neurites longer than 150 μm was calculated.
Immunostaining of cultured cells
The cultured cells were mildly fixed for 10 min by adding the same amount of 4% PFA solution as culture medium, and post-fixed with the same fixative for 10 min at room temperature. After having been washed with PBS, the cells on the coverslips were incubated for 15 min at 37°C in 0.1-M Tris-HCl buffer (pH 7.4) containing 0.3% (v/v) Triton X 100, to make the cell membranes permeable. After another wash with PBS, the cells were treated with 2% Block Ace for 30 min at room temperature, and then reacted with diluted primary antibodies at 4°C overnight. After the cells had been washed with PBS, they were incubated with fluorescence-conjugated secondary antibodies or biotinylated secondary antibody for 3 h at room temperature. For the avidin-biotin complex (ABC) method, the cells were incubated in avidin-biotin- antiperoxidase complex (Vector Laboratories), and the enzyme activity was visualized with diaminobenzidine (Dojin Laboratories, Kumamoto, Japan), with H2O2 as the substrate. The following primary antibodies were used for this analysis: rabbit anti-fibronectin (1:1000; LSL), monoclonal mouse anti-βIII-tubulin (1:1000; Promega), mouse anti-NG2 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-N-cadherin (1:500; BD Transduction Laboratories, Becton Dickinson and Company). The secondary antibodies used were: Alexa 488-conjugated goat anti-mouse IgG, and Alexa 546-conjugated goat anti-rabbit IgG, (1:1000, both from Invitrogen), and biotinylated anti-mouse IgG (1:1000, Vector Laboratories).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from confluent cultures of MDFs and FIFs using Trizol Reagent (Invitrogen) according to the manufacturer's instructions. All RNA samples were treated with DNase I to remove contaminating genomic DNA. Semi-quantitative RT-PCR was performed as described previously28 to assess mRNA levels of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), neuregulin-1, N-cadherin, VE-cadherin, thrombomodulin, and vascular endothelial growth factor receptor (VEGFR)-1. β-Actin was used as internal control. Briefly, a 20-μL volume of PCR reaction contained 1 μL of first-strand cDNA, 4 μL of 5×Go Taq Flexi Buffer (Promega), 1.25 U of Go Taq DNA polymerase (Promega), 2-mM MgCl2, 0.2 mM of each of the four deoxynucleotide triphosphates, and 0.1 μmol of each specific primer. The amplification was carried out with a thermal cycler at 94°C for 5 min, followed by 24–38 cycles consisting of 94°C for 30-sec, 60–65°C for 1 min, and 72°C for 45 sec. The sequences of primers, annealing temperatures, and size of PCR products were: β-actin forward primer, 5′-GCCGTC TTCCCCTCCATCGT-3′, and reverse primer, 5′-CCCGTC TCCGGAGTCCATCA-3′, 63°C, 390 bp; NGF forward primer, 5′-GGGGATCCTCCACCCACCCAGTCTTCCAC-3′, and reverse primer, 5′-GGCAAGTCAGCCTCTTCTTGTAGCCT TCC-3′, 60°C, 376 bp; BDNF forward primer, 5′-CCCAGGG CAGGTTCGAGAGG-3′, and reverse primer, 5′-CCCGCCA GACATGTCCACTG-3′, 61°C, 350 bp; GDNF forward primer, 5′-GAGAGGAATCGGC-AGGCTGCAGCTG-3′, and reverse primer, CAGATACATCCACATCGTTTAGCGG-3′, 60°C, 337 bp; neuregulin-1 forward primer, 5-CCCCTCCTGGGAC AAACTTTTCTG-3′, and reverse primer, 5′-GCATGCCA-GTGATGAACTCGTTTG-3′, 63°C, 517 bp; N-cadherin forward primer, 5′-AGCCCCACA-CCCTGGGGATA-3′, and reverse primer, 5′-TTCGCAGCCTACGCCAAAGC-3′, 63°C, 383 bp; VE-cadherin forward primer, 5′-GCAAGACCAGTG ACAGAGGCCAAT-3′, and reverse primer, 5-GATGTTGGC GGTATTGTCGTGGTT-3′, 63°C, 383 bp; thrombomodulin forward primer, 5-TCGCGCAGAGCTGGAGTACAAGTG-3′, and reverse primer, 5-AGGTTCGGGGAGGAGAATGAT GGA-3′, 65°C, 580 bp; and VEGFR-1 forward primer, 5′-AAATCACTCGGGAGAGGGGCTTTT-3′, and reverse primer, 5-CCAGGTCTGGCTCCAGCTTTTCTT-3′, 63°C, 358 bp. After amplification, an aliquot of the PCR products was subjected to 2% agarose gel electrophoresis and visualized by ethidium bromide staining. The images of the bands were captured on FLA-5100 (Fujifilm, Tokyo, Japan). The optical density of each band was quantified by utilizing image-analysis software (ImageJ; National Institutes of Health). We carefully checked the reliability of the RT-PCR used in this study. That is, the quantification was performed within the range of linearity between the amount of RNA added and the obtained intensity of the band of a particular PCR product.
Cell transplantation
FIFs from individual animals (n=9), or MDFs from individual animals (n=5), were grown to near confluence in 10-cm culture dishes and then labeled with the nuclear dye bis-benzimide (Hoechst 33342; Invitrogen), by adding 3-μL of a 10-mg/mL solution of the dye to 6 mL of culture medium. In 2 h, the Hoechst 33342 dye-labeled FIFs or MDFs were thoroughly washed with PBS, detached from the culture vessels by treatment with 0.1% trypsin, and centrifuged. The pellet was resuspended in DMEM containing 10% FBS at a density of 106 cells/10 μL.
The spinal cord was completely transected as described above, and followed by FGF-2 administration. As indicated above, the distal stump was carefully lifted up to confirm complete transection, and we ensured that the distal and proximal stumps came into contact when the former stump was returned to its original position. Immediately after surgery, FIFs or MDFs were transplanted into the gap between the rostral and caudal spinal cord stumps. After transplantation, the muscles and skin were sutured, and the animals were placed in standard cages and given free access to food and water. No immunosuppressants were used in this study.
Statistical analysis
All numerical data are presented as group mean values with standard deviation. Statistically significant differences of various gene expressions in MDFs and FIFs were determined using the Student's t test. Those in the experiments of retrograde axonal transport using FG, identification of the proliferating cells in the injured spinal cord using BrdU, and neuritegenesis by the cells, were evaluated with one-way analysis of variance (ANOVA) with Tukey's post hoc test. Statistical analysis of locomotor function as assessed by the BBB scale or inclined plane test was performed by repeated-measures two-way ANOVA, followed by the Bonferroni post-test.
Results
FGF-2 improves locomotor function after SCI
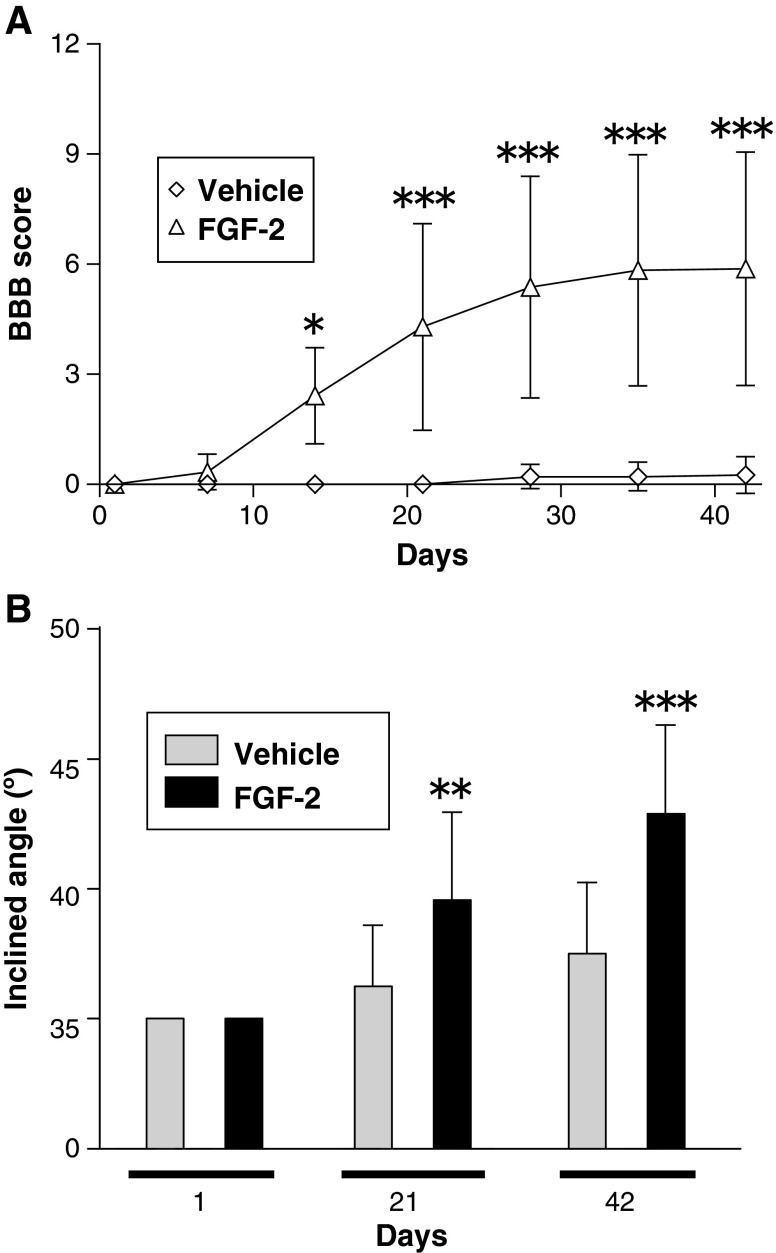
Locomotor function was assessed by use of the BBB locomotor rating scale, a commonly used method of assessing motor function after SCI.26 All injured rats displayed complete hindlimb paralysis immediately after injury. In the vehicle-treated group, no improvement was observed throughout the experimental period of 6 weeks (i.e., the rats remained in complete paralysis). In contrast, from 4–6 weeks, the FGF-2-treated rats attained nearly 6 points on the BBB scale, which meant that they could move two of their hindlimb joints well, but could only slightly move the third joint. A two-way ANOVA revealed a significant effect of FGF-2 treatment [F(1,77)=136.7; p<0.001], and a significant interaction between FGF-2 treatment and days after injury/treatment [F(1,6)=16.22; p<0.001]. Post-hoc analyses showed that the FGF-2 treatment led to significantly higher BBB scores than vehicle-treatment (p<0.05 for 14 days, and p<0.001 for 21, 28, 35, and 42 days after injury; Fig. 1A). These observations suggest that FGF‐2 markedly ameliorated SCI-induced hindlimb paralysis.
FIG. 1.
Fibroblast growth factor-2 (FGF-2) injection into the injured spinal cord improves locomotor function. (A) Time course of the locomotor function of the vehicle-treated (n=12) and FGF-2-treated animals (n=12) during the 6-week experimental period. The FGF-2-treated group showed a significant improvement over the vehicle-treated group. (B) Locomotor function was tested by use of the inclined plane test on the same groups of animals shown in A. Spinal cord injury caused locomotor loss in this test, but FGF-2 treatment ameliorated it. The FGF-2-treated rats could climb the board more steeply inclined than could the vehicle-treated rats (*p<0.05, **p<0.01, ***p<0.001 versus the vehicle-treated group; BBB, Basso, Beattie, and Bresnahan locomotor scale).
Improvement of locomotor activity was also evaluated by another method, the inclined plane test, which assesses the maximal angle at which an animal can support its body weight on an inclined board. Normal rats support their body with both forelimbs and hindlimbs; however, the injured rats could use only their forelimbs; therefore injured animals were prone to slip on such a steeply inclined board. A two-way ANOVA analysis revealed a significant effect of FGF-2 treatment [F(1,33)=24.01; p<0.001], and a significant interaction between FGF-2 treatment and the days after injury/treatment [F(1,2)=8.869; p<0.001]. Post hoc analysis showed significantly higher inclined plane scores with FGF-2 treatment than with PBS treatment (p<0.01 for 21 days, and p<0.001 for 42 days after injury; Fig. 1B). These observations support the above results, indicating that FGF-2-treatment facilitated recovery from SCI-induced locomotor dysfunction.
FGF-2 facilitates retrograde transport of descending pathways
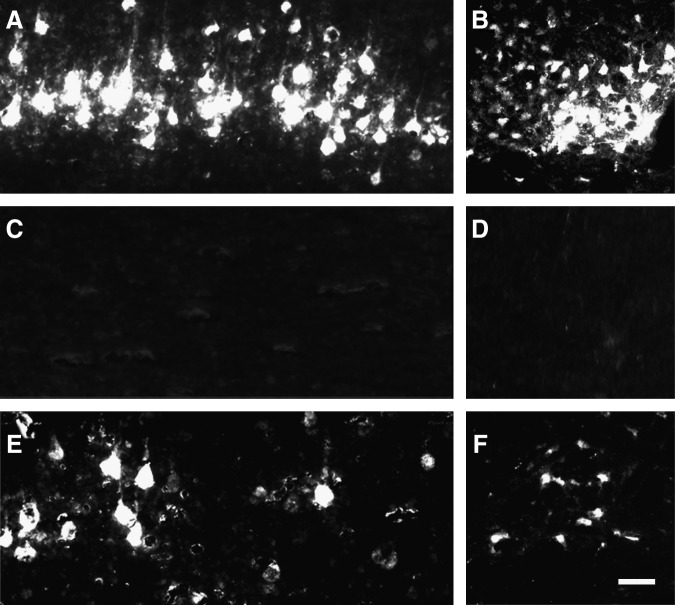
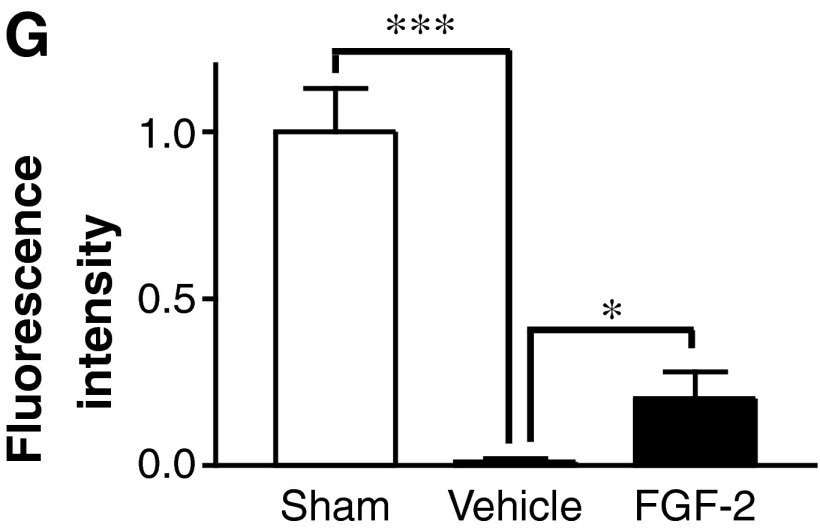
To examine whether FGF-2 was capable of inducing regeneration of spinal motor pathways such as the corticospinal and rubrospinal tracts (RSTs), we traced the retrograde axonal transport of the FG that had been injected caudal to the transection site into the sensory motor cortex and red nucleus. Numerous FG fluorescence-positive cells were detected histochemically in the sham-operated rats, whereas no positive cells were seen in the SCI animals without FGF-2 treatment, demonstrating the efficacy of this experimental protocol (Fig. 2). FG-positive cells were detected in the sensory motor cortex or red nucleus of the SCI animals with FGF-2 treatment (Fig. 2E and F), suggesting that FGF-2 facilitated regeneration of the corticospinal tract or RST. However, the number was considerably lower in the retrograde targets of the FGF-2-treated SCI rats than in those of the sham-operated animals. It is notable that the intensity of fluorescence in the positive cells was similar between the sham-operated animals and the SCI animals with FGF-2 treatment. This may indicate that active transport of FG occurred substantially but limitedly in the regenerating axons. Thus the amounts of FG transported into the sensory motor cortex were quantitatively evaluated. The intensity of the fluorescence signal extracted from the sensory motor cortex of the SCI animals was markedly lower regardless of treatment, compared with that extracted from the corresponding tissues of the sham-operated animals (Fig. 2G; p<0.001). However, the fluorescence intensity extracted from the tissues was significantly different between the vehicle-treated and FGF-2-treated SCI groups (Fig. 2G; p<0.05). These results suggest that FGF-2 facilitated regenerative axonal growth in the corticospinal tract and/or RST.
FIG. 2.
Fibroblast growth factor-2 (FGF-2) facilitates retrograde transport of descending pathways. (A–F) Fluorescence photomicrographs of the sensory motor area of the cerebral cortex (A, C, and E), and red nucleus (B, D, and F), from the sham-operated (A and B), vehicle-treated (C and D), and FGF-2-treated (E and F) groups. Note that a small number of fluorogold (FG)-labeled neurons was observed in the FGF-2-treated animals. (G) Intensity of fluorescence of the FG extracted from the sensory motor area. The intensity in the FGF-2-treated group (n=4) was significantly higher than that of the vehicle-treated group (n=4). The mean value of the sham-operated group (n=3) is expressed as 1.0 (*p<0.05, ***p<0.001 versus the vehicle-treated group; scale bar=50 μm).
FGF-2 induces a mass of fibronectin-positive cells in the lesion site
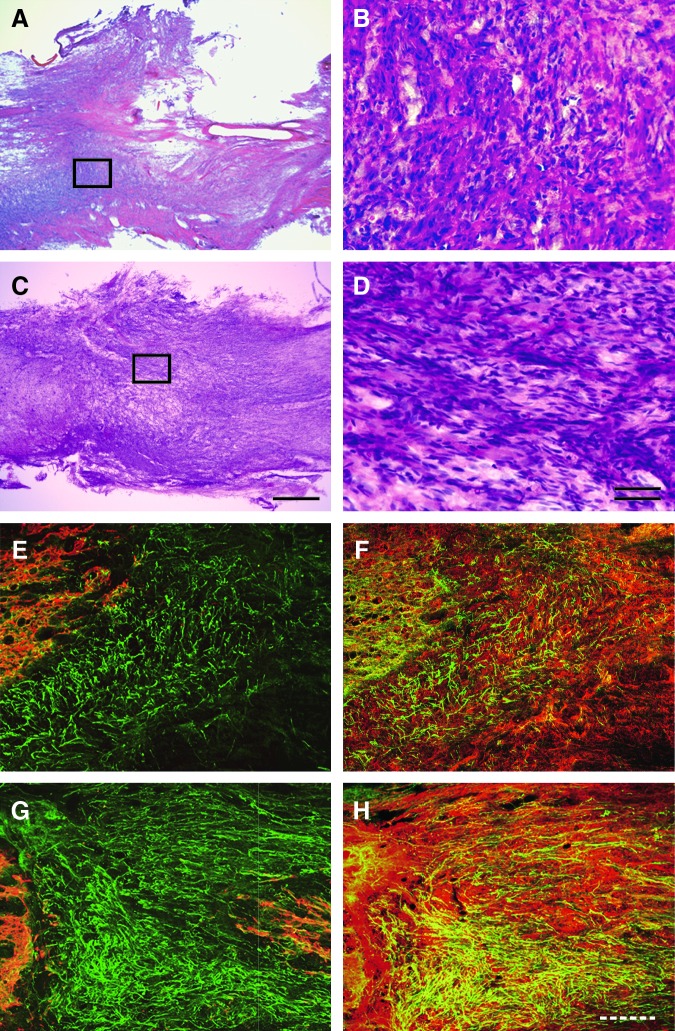
There were large cavities and tissue loss in the lesion site of the vehicle-treated SCI group 6 weeks after SCI (Fig. 3A). In the rest of the lesion site, intrinsic cells had partly filled up the space (Fig. 3B). In contrast, in the FGF-2-treated SCI group, newly generated tissues 1–2 mm in length completely filled the cavity of the lesion site, bridging the rostral and caudal stumps (Fig. 3C and D). These observations suggest that some intrinsic cells had proliferated and/or gathered at the lesion site in response to the FGF-2, which may help nerve regeneration.
FIG. 3.
Fibroblast growth factor-2 (FGF-2) injection around the injury site of the spinal cord induces a mass of fibronectin-positive cells that aid in axonal regeneration. Gross appearance of the spinal cord around the injury site of the vehicle-treated (A) and FGF-2-treated (C) rats on experimental day 42 are shown, with the boxed areas in A and C enlarged in B and D, respectively. Fluorescence photomicrographs of longitudinal sections through the spinal cords of the vehicle-treated (E and F) and FGF-2-treated (G and H) groups are also shown. Sections immunostained for growth-associated protein-43 (GAP-43; green) and glial-fibrillary acidic protein (GFAP; red) are merged (E and G), as are those for GAP-43 (green) and fibronectin (red; F and H). Note that more regenerated axons traversed the mass of fibronectin-positive cells in the FGF-2-treated group than in the vehicle-treated group. The left side is rostral (scale bars in A and C=500 μm; in B and D=50 μm, double line; in E–H=200 μm, dashed line). Note that both stumps are strongly labeled with GFAP.
To clarify the neuroregenerative actions of FGF-2, we next examined the distribution of particular neuronal antigens in the injured spinal cord by using an immunohistochemical technique. A few axons with growth-associated protein-43 (GAP-43) immunoreactivity expanded irregularly within the borders of the lesions as shown by GFAP labeling in the vehicle-treated group (Fig. 3E); however, a substantial number of axons positive for GAP-43 crossed, in a rostrocaudal direction, the newly generated tissues that had filled the cavity of the lesion site in the FGF-2-treated group (Fig. 3G). We further visualized the immunoreactivity of fibronectin in the same way, and observed an abundance of fibronectin‐positive cells in the epicenter of the lesion site of both the vehicle-treated and FGF-2-treated groups (shown in red in Fig. 3F and H). Surprisingly, many GAP-43-positive axons penetrated into the intercellular space occupied by fibronectin-positive cells in the FGF-2-treated group (Fig. 3H). These observations demonstrate that fibronectin-positive cells induced by FGF-2 filled the cavity of the lesion site and may have contributed to axonal regeneration after SCI.
Distribution of the cells responsive to FGF-2
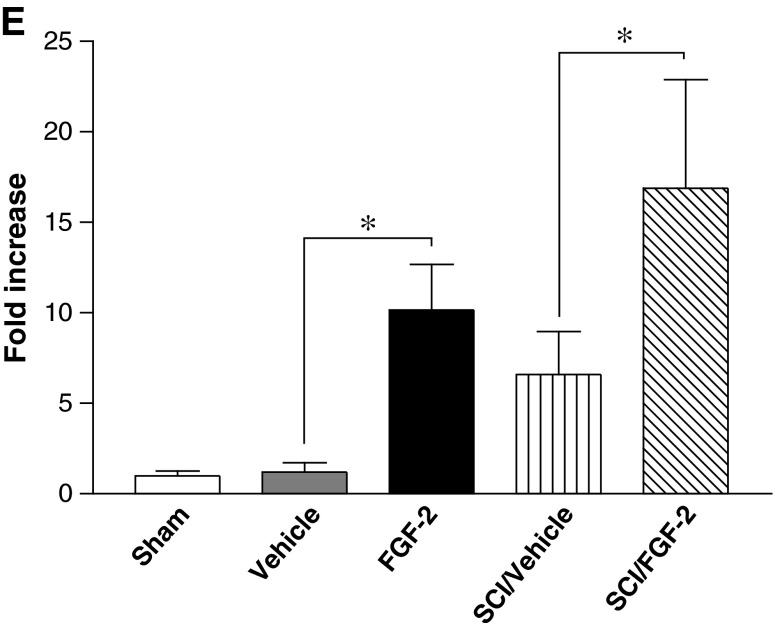
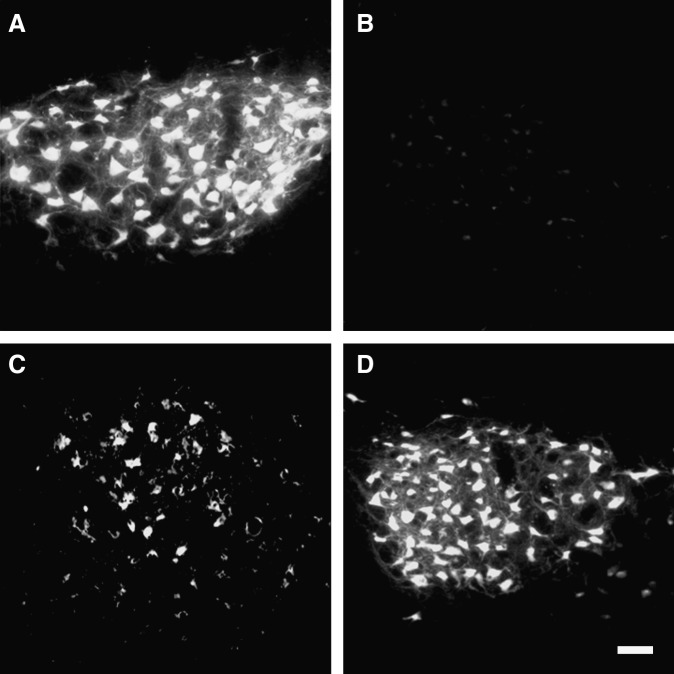
To assess the origin of the fibronectin-positive cells induced by FGF‐2, we estimated the influences of FGF-2 and/or SCI on the mitotic activity of cells of the injured spinal cord. BrdU was injected IP into rats with or without SCI and/or FGF‐2 administration, and then the number of BrdU-positive cells was counted 2 days later. FGF‐2 markedly increased the number of BrdU-positive cells; however, SCI also slightly elevated the numbers (Fig. 4).
FIG. 4.
Fibroblast growth factor-2 (FGF-2) injected into the inner spinal cord induces mitotic activity. Fluorescence photomicrographs depicting cells that had incorporated 5′-bromo-2′-deoxyuridine (BrdU) into the uninjured (A and B) or injured spinal cords (C and D), injected with vehicle (A and C), or FGF-2 (B and D) are shown. The number of BrdU-positive cells was significantly higher in the FGF-2-treated rats, regardless of injury (E; SCI, spinal cord injury; Sham, sham-operated animals; Vehicle, vehicle-treated uninjured animals; FGF-2, FGF-2-treated uninjured animals; SCI/Vehicle, vehicle-treated injured animals; SCI/FGF-2, FGF-2-treated injured animals; *p<0.05 versus the corresponding vehicle-treated group; scale bar=20 μm).
To identify the cell type responsive to FGF-2, we doubly immunostained the tissues for both BrdU and each of various cell markers. The percentage of BrdU‐positive cells with fibronectin immunoreactivity (BrdU+/fibronectin+) was markedly increased by FGF‐2, regardless of injury (Fig. 5A); whereas that of BrdU-positive cells expressing NG2 (a marker for oligodendrocyte precursor cells; BrdU+/NG2+) was significantly decreased by injury, but not by FGF-2 (Fig. 5B). The percentage of BrdU‐positive cells expressing GFAP (a marker of astrocytes; BrdU+/GFAP+) was not changed by injury and/or FGF-2 (Fig. 5C). These results suggest that FGF-2 selectively stimulated proliferation of fibronectin-positive cells. The percentage of BrdU-positive cells expressing OX-42 (a marker for macrophages and microglia; BrdU+/OX-42+) dramatically increased after SCI; however, it remained low in the FGF-2-treated SCI rats (Fig. 5D). FGF-2 may suppress the proliferation of SCI-induced OX-42-positive cells, or the FIFs may participate in the expulsion of these cells from the epicenter of the lesion site.
FIG. 5.
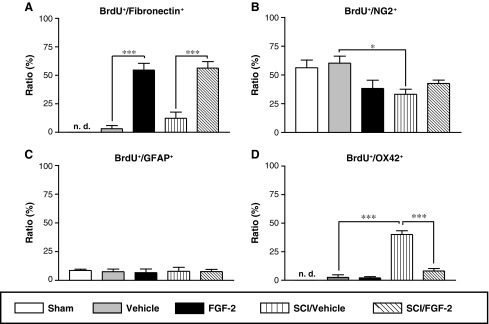
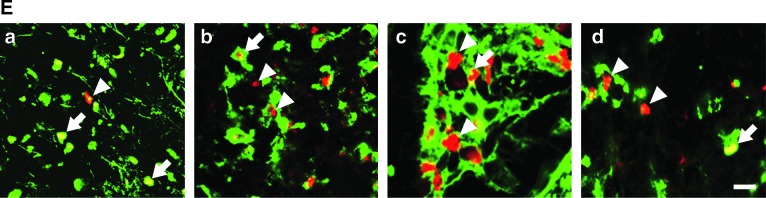
Fibronectin-positive cells respond to exogenous fibroblast growth factor-2 (FGF-2), but not to spinal cord injury (SCI). (A–D) The ratios of cell marker–expressing BrdU-positive cells to the total number of BrdU-positive cells were calculated for the injured or uninjured spinal cords after vehicle or FGF-2 injection. (A) FGF-2 administration significantly increased the ratio of fibronectin-expressing BrdU-positive cells (BrdU+/fibronectin+), regardless of injury. (B) SCI decreased the ratio of BrdU+/NG2+ cells, but FGF-2 did not. (C) Neither SCI nor FGF-2 affected the ratio of BrdU+/GFAP+ cells. (D) SCI caused a marked increase in the ratio of BrdU+/OX-42+, but the increment was attenuated by FGF-2 administration. (E) Fluorescence photomicrographs illustrate typical patterns of BrdU+/fibronectin+ (a), BrdU+/NG2+ (b), BrdU+/GFAP+ (c), and BrdU+/OX-42+ cells (d) observed in the FGF-2-treated injured spinal cord. Sections were stained for BrdU (red) or cell markers (green). Arrowheads and arrows indicate BrdU-positive cells without cell marker staining and BrdU-positive cells expressing cell marker antigen, respectively (scale bar=10 μm; *p<0.05, ***p<0.001 for the difference between the bracketed values; Sham, sham-operated animals; Vehicle, vehicle-treated uninjured animals; FGF-2, FGF-2-treated uninjured animals; SCI/Vehicle, vehicle-treated injured animals; SCI/FGF-2, FGF-2-treated injured animals; BrdU, 5-bromo-2′-deoxyuridine; GFAP, glial-fibrillary acidic protein).
Only a limited number of cells bearing βIII-tubulin (a marker for neurons) appeared in the injured spinal cord and only when the injury site was treated with FGF-2 (data not shown). These observations would seem to preclude the possibility that FGF-2 stimulated the proliferation of neural stem cells present along the central canal.
Preliminary observations about the therapeutic effects of FGF-2 described above were previously reported.29
Primary culture of FGF-2–induced cells
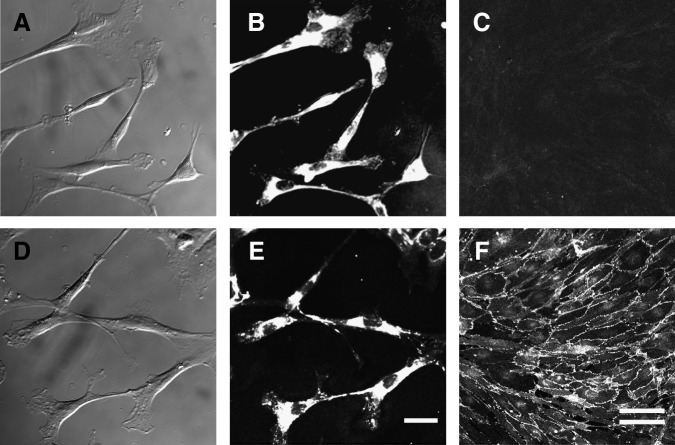
The results of the histochemical analysis raised the possibility that FGF-2-induced fibronectin-positive cells around the injury site may have played important roles in the axonal regeneration seen after SCI. As meningeal cells are fibroblast-like cells expressing fibronectin, they are one of the possible candidates. However, meningeal cells form a very poor substrate for growing neurites, and are thought to form a physical barrier to inhibit penetration by regenerating nerves.30 Therefore, to compare these two types of fibronectin-positive cells, we prepared primary cultures of the FIFs and MDFs as described in the methods section. In both FIF and MDF cultures, which were grown in DMEM supplemented with FGF-2 and 10% FBS, spindle-shaped fibroblast-like cells emerged around the explanted tissues and migrated out, proliferating 2 days after the start of the cultures (Fig. 6A and D). Both cultures reached confluence within 1 week, and the cells in each strongly expressed fibronectin (Fig. 6B and 6E). The FIFs heavily expressed N-cadherin on their cell surface, one of the molecules favorable for neuritegenesis,31,32 whereas the MDFs did not (Fig. 6C and 6F), demonstrating that FIFs are a cell population different from MDFs, and that MDFs had not contaminated the FIF cultures.
FIG. 6.
Morphological and immunocytochemical analyses of FGF-2-induced fibronectin-positive cells (FIFs). Phase-contrast images of meninges-derived fibronectin-positive cells (……MDFs) (A) and FIFs (D) show that both types of cells are spindle-shaped and fibroblast-like. As shown in the fluorescence photomicrographs of MDFs (B and C) and FIFs (E and F), fibronectin was expressed in all of the MDF (B) and FIF (E) cells. N-cadherin was expressed in cell-to-cell adhesion regions of FIFs (F), but not in those of MDFs (C; scale bars in A, B, D, and E=50 μm; those in C and F=100 μm, double line; FGF-2, fibroblast growth factor-2).
Effects of FIFs and MDFs on neuritegenesis in vitro
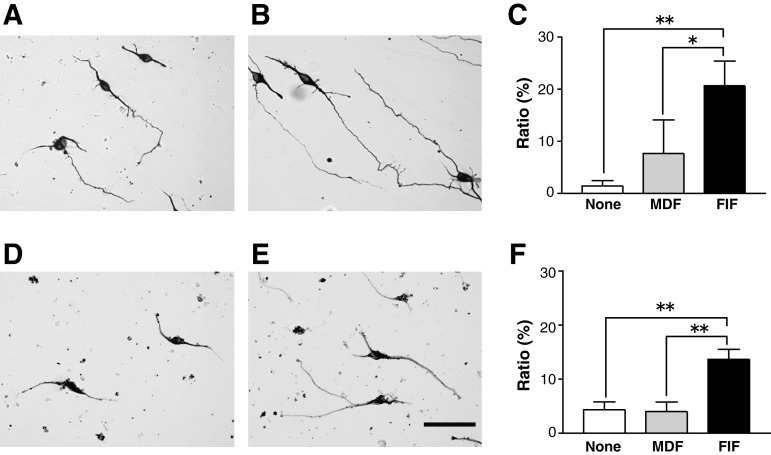
How did the FIFs facilitate nerve regeneration? It is conceivable that neuritegenesis is facilitated by diffusible agents secreted from feeder cells in addition to active molecules expressed on the plasma membrane of the feeder cells. Mouse cortical neurons were cultured on monolayers of FIFs or MDFs for 2 days, and the percentage of neurons (positive for βIII-tubulin antigen) with neurites longer than 150 μm was calculated. The percentage of neurons with long neurites was much higher when the neurons were cultured on the FIFs than on the MDFs (Fig. 7A–C), suggesting that the FIFs secreted diffusible molecules and/or expressed membrane‐bound molecules that help facilitate neuritegenesis. To discriminate the effects of diffusible factors from those of membrane-bound molecules, we cultured cortical neurons in the medium conditioned for 2 days by FIFs or MDFs. The results showed that the percentage of neurons extending long neurites was significantly higher when the conditioned medium by FIFs was used (Fig. 7D–F), suggesting that the FIFs facilitated neuritegenesis at least in part by secreted diffusible agents such as neurotrophic factors. However, the contribution of the membrane-bound molecules to neuritegenesis was unclear based on these experiments. This experiment was repeated three times independently, and similar results were obtained for all.
FIG. 7.
FGF-2-induced fibronectin-positive cells (FIFs) promote neurite extension through cell-cell interactions and secreted diffusible factors. (A–C) A typical pattern of cortical neurons cultured for 2 days on the monolayer sheet of meninges-derived fibronectin-positive cells (……MDFs) (A) or FIFs (B), and stained for βIII-tubulin antigen. The percentages of neurons with neurites longer than 150 μm are shown (C). (D–F) A typical pattern of cortical neurons cultured for 2 days in medium conditioned by MDFs (D) or FIFs (E), and stained as described above, with the percentage of neurons having neurites longer than 150 μm also shown (F). Note that the percentages were significantly higher when the neurons were cultured on the FIF monolayers or in the medium conditioned by FIFs (*p<0.05, **p<0.01 for difference between the bracketed values; scale bar=50 μm; FGF-2, fibroblast growth factor-2).
Gene expression profile of FIFs and MDFs
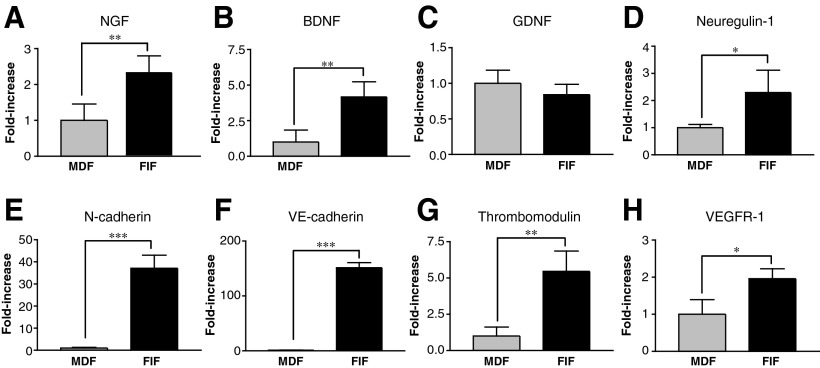
To clarify the molecular basis underlying the facilitation of neuritegenesis by FIFs, we performed RT-PCR to evaluate the gene expression of cell-adhesion molecules, several neurotrophic factors, and various molecules useful for cell typing. The gene expression level of neurotrophic factors such as NGF, BDNF, and neuregulin-1 was higher in FIFs than in MDFs, but the levels of GDNF mRNA were similar (Fig. 8A–D). Further, mRNA expression of N-cadherin, a cell-adhesion molecule expressed on neurons and mesenchymal cells, was conspicuously upregulated in the FIFs (Fig. 8E). This result is consistent with the previous result that the N-cadherin molecule was strongly expressed in cultured FIFs, particularly on their cell surface (Fig. 6F). VE-cadherin, thrombomodulin, and VEGFR-1 are molecules that characterize vascular endothelial cells, and their genes were expressed at higher levels in FIFs than in MDFs, suggesting a putative origin of the FIFs (Fig. 8F–H).
FIG. 8.
Reverse transcription polymerase chain reaction (RT-PCR) analysis of the expression of various genes in FGF-2-induced fibronectin-positive cells (FIFs) and meninges-derived fibronectin-positive cells (……MDFs). (A–D) mRNA expression levels of nerve growth factor (NGF) (A), brain-derived neurotrophic factor (BDNF) (B), glial cell line-derived neurotrophic factor (GDNF) (C), neuregulin-1 (D), and N-cadherin (E) are illustrated. (F–H) mRNA expression levels of VE-cadherin (F), thrombomodulin (G), and vascular endothelial growth factor receptor (VEGFR-1) (H), all of which are markers for vascular endothelial cells, are shown. The ratio of the expression level in MDFs of each gene to that of β-actin was averaged, and the mean value was considered as 1.0 (*p<0.05, **p<0.01, ***p<0.001 versus the value for MDF; FGF-2, fibroblast growth factor-2).
Transplantation of FIFs helps alleviate locomotor dysfunction
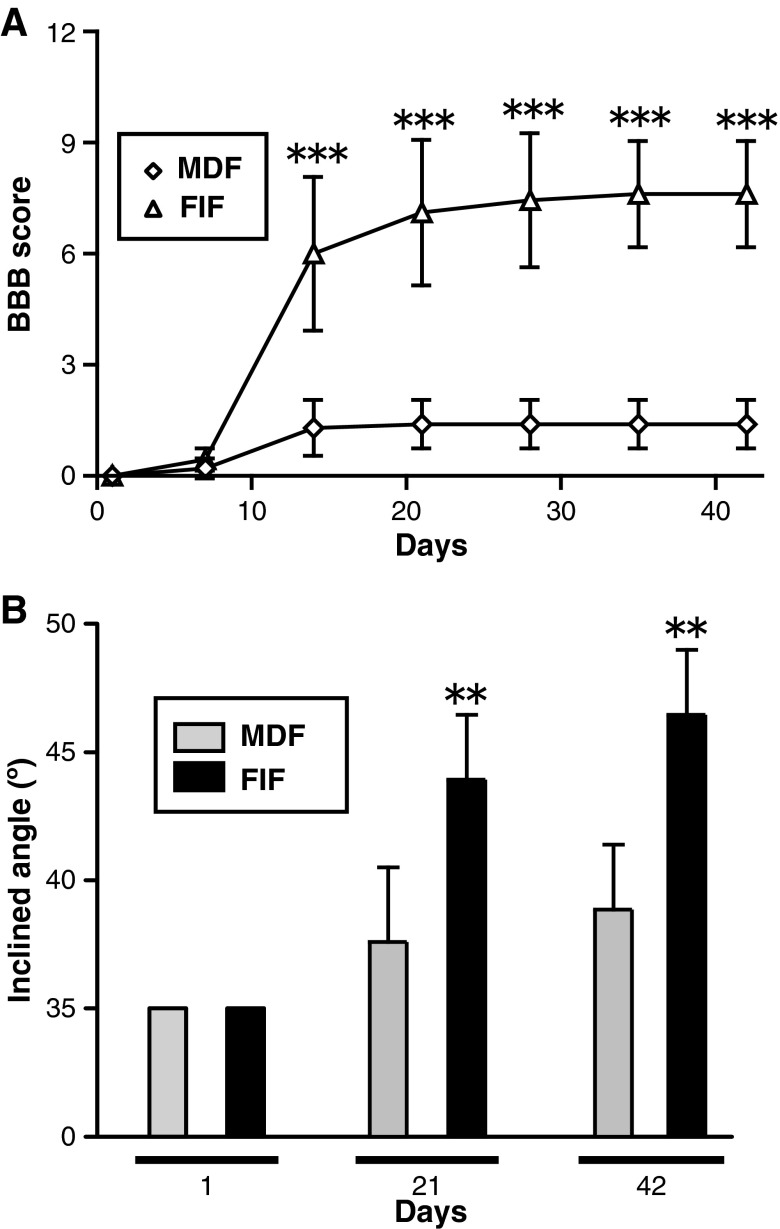
We found that FIFs strongly expressed several genes favorable for neurite growth, which prompted us to assess the ability of the FIFs to alleviate locomotor dysfunction after complete SCI. From approximately 3–6 weeks, the FIF-transplanted rats attained over 7 points on the BBB scale, which meant that they could extensively move two of their hindlimb joints and somewhat move their third joint (Fig. 9A). Two-way ANOVA revealed a significant effect for FIF transplantation [F(1, 72)=51.99; p<0.001], and a significant interaction between FIF transplantation and days after injury/transplantation [F(1, 6)=40.89; p<0.001]. Post-hoc analysis showed significantly higher BBB scores with FIF transplantation than with MDF transplantation (p<0.001 for 14, 21, 28, 35, and 42 days post-injury; Fig. 9A). The inclined plane test was performed to assess the locomotor function in the cell-transplanted animals, as it had been used for the FGF-2-treated animals (Fig. 1). Again, two-way ANOVA revealed a significant effect for FIF transplantation [F(1,9)=28.66; p<0.001], and a significant interaction between FIF transplantation and days after injury [F(1,2)=6.975; p<0.05]. Post-hoc analysis showed significantly higher inclined plane scores with FIF transplantation than with MDF transplantation (Fig. 9B; p<0.01 for 21 and 42 days after injury). These results strongly support the idea that the FIFs, which proliferated and occupied the space in the lesion site, had the ability to ameliorate locomotor function after complete SCI.
FIG. 9.
Transplantation of FGF-2-induced fibronectin-positive cells (FIFs) into the injured spinal cord improves locomotor function of the animals. (A) Time course of locomotor function during the 6-week period after the transplantation of meninges-derived fibronectin-positive cells (……MDFs) (n=5) or FIFs (n=9) into the transection site. The FIF-transplanted group showed a significant improvement compared with the MDF-transplanted group. (B) Locomotor function was also tested using the inclined plane test on the same group of animals. The FIF-transplanted rats could climb a more steeply inclined board than the MDF-transplanted ones (**p<0.01, ***p<0.001 versus the values for the MDF-transplanted group; FGF-2, fibroblast growth factor-2; BBB, Basso, Beattie, and Bresnahan score).
Transplantation of FIFs facilitates retrograde axonal transport of FG in the RST
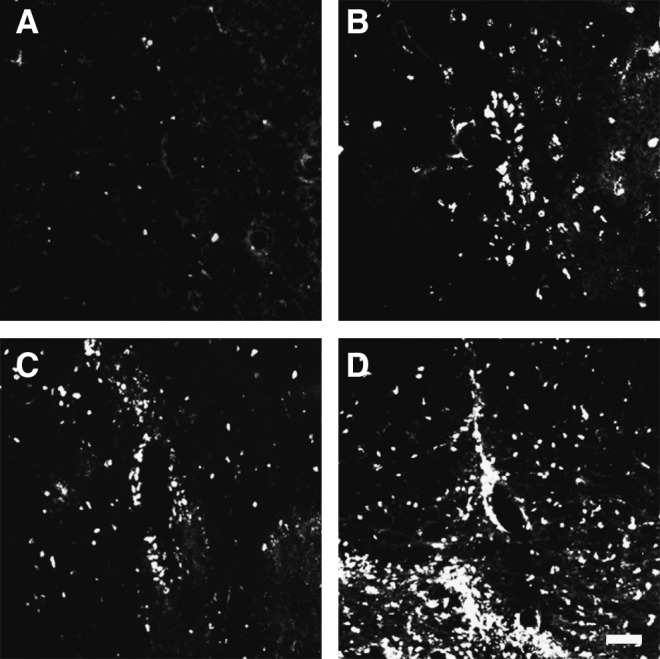
To address how the FIFs could improve the SCI-induced decrease in locomotor activity, we assessed the axonal regeneration of the RST by using FG, which is retrogradely transported in axons from the axonal terminal to the neuronal cell body. The FG suspension was injected into the spinal cord 5-mm caudal to the injury site 42 days after SCI, and FG was similarly injected into the spinal cords of the sham-operated animals and the non-transplanted animals with lesions at the position corresponding to the injury site of the experimental animals. The fluorescent signal in the red nucleus was then analyzed. Many cells were heavily labeled in the sham-operated animals (Fig. 10A); in contrast, no labeled cells were seen in the non-transplanted SCI animals (Fig. 10B). Only a few labeled cells were seen in the MDF-transplanted SCI rats (Fig. 10C), whereas a substantial number of positive cells was observed in the FIF-transplanted SCI rats (Fig. 10D). These results suggest that transplanted FIFs induced regenerative axonal growth of the RST, and thereby facilitated the functional recovery of SCI-induced locomotor dysfunction.
FIG. 10.
Transplantation of FGF-2-induced fibronectin-positive cells (FIFs) facilitates retrograde axonal transport of fluorogold (FG) in the rubrospinal tract (RST). (A–D) Fluorescence photomicrographs of representative sections of the red nucleus from the sham-operated (A), vehicle-treated (B), meninges-derived fibronectin-positive cells (……MDF)-transplanted (C), and FIF-transplanted (D) groups. Note that a substantial number of FG-labeled neurons were seen in the FIF-transplanted animals (scale bar=50 μm; FGF-2, fibroblast growth factor-2).
Histochemical analysis of nerve regeneration in the transplanted graft
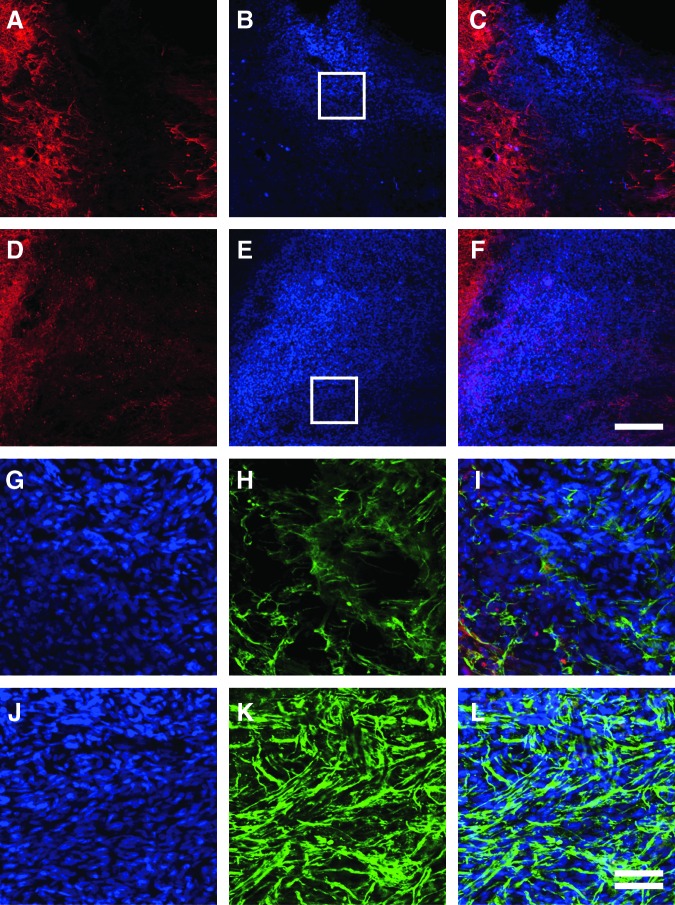
The effect of cell transplantation on nerve regeneration was evaluated by immunohistochemical analysis. Cells were pre-labeled with Hoechst 33342, and transplanted into the injury site. Both types of cells (FIFs and MDFs) survived well, filled in the cavity, and were closely apposed to the host tissue when observed 42 days after transplantation (Fig. 11A–F). Astrocytes (GFAP-positive cells) of the host tissue surrounded the grafts in the case of both FIFs and MDFs (stained with Hoechst 33342), but did not invade into the graft tissue. Numerous regenerating axons with GAP-43 immunoreactivity penetrated into the FIF grafts (Fig. 11H–I), whereas fewer axons entered the MDF grafts (Fig. 11K–L). The MDFs seemed to have repulsively interacted with the generated neurites, because these neurites showed a bias against the graft. These results indicate that the FIFs effectively stimulated nerve regeneration, and that the generated neurites passed through the graft, which might result in improvement of SCI-induced locomotor dysfunction.
FIG. 11.
Transplantation of FGF-2-induced fibronectin-positive cells (FIFs) around the injury site of the spinal cord promotes axonal regeneration. Fluorescence photomicrographs of the host spinal cord were taken 6 weeks after the transplantation of meninges-derived fibronectin-positive cells (……MDFs) (A–C and G–I), or FIFs (D–F and J–L). (A–F) Immunoreactivity of glial-fibrillary acidic protein (GFAP) of the host (A and D, red) and Hoechst dye-stained graft (B and E, blue) are merged (C and F). Note that GFAP signal and Hoechst dye staining are observed in separate sites, and that MDFs and FIFs survived well in the host tissue. (G–L) Hoechst staining (G and J, blue), and immunoreactivity of growth-associated protein-43 (GAP-43) (H and K, green), are merged in I and L. Boxed areas in B and E are enlarged in G–I and J–L, respectively. Note that extremely abundant regenerative axons coursed through the transplanted FIFs. The left side is rostral (scale bars in C and F=200 μm, and in I and L=50 μm, double line; FGF-2, fibroblast growth factor-2).
Discussion
The present study demonstrated that FGF-2 administration into the spinal cord dramatically enhanced locomotor functional recovery after complete transection of the spinal cord in rats. After 4–6 weeks the BBB scores of the FGF-2-treated group were nearly 6, which indicated that the injured animals could extensively move two of their hindlimb joints, but only slightly move the third joint. This effect was more marked than expected, because FGF-2 administration via a minipump was shown to be significantly effective for functional recovery, but its effect was not so great for a contusion injury.20,21 The method for administration of FGF-2 seems to be important for the efficacy of amelioration of locomotor activity. Infusion of FGF-2 with a minipump into the medullary space of the spinal cord or injection of collagen gel containing FGF-2 into the space adjacent to the injury site are methods for long-lasting application of FGF-2. However, these methods induced only a slight improvement in locomotor activity.21,33,34 Jimenez Hamann and colleagues33 administered FGF-2 and EGF in combination into the medullary space of the spinal cord and found that the distribution of FGF-2 in the spinal cord was quite different from that of EGF. Namely, EGF had penetrated the spinal cord 30 min after administration, but FGF-2 was only present in the pia and dura mater, but not in the spinal cord beneath the pia, at all time points analyzed from 30 min to 7 days. In this case the administered FGF-2 failed to activate the cells needed for neural regeneration. Therefore in the present study, FGF-2 was directly injected into the spinal cord tissue.
As there are no reports analyzing the action of FGF-2 after injection into the parenchyma of the spinal cord, we compared our results with those achieved by local injection of FGF-2 into the adult rat brain, as described by Gonzalez and associates.35 They found that FGF-2 was not only stored in extracellular matrix, but that it was specifically transferred and then internalized by specific cell populations, after being actively concentrated over the target tissues. Further, the 18-kDa FGF-2 could still be detected after 4 and 7 days in the rat brain. These findings support the idea that the matrix can act as a long-term storage site that subsequently delivers a biologically active form of FGF-2. It is likely that exogenously administered FGF-2 is similarly stored and transferred in the spinal cord, which may be why FIFs proliferate around the injection site.
Invading meningeal cells generally produce a fibroblastic scar after damage to the spinal cord, and this scar forms an absolute barrier to axonal regeneration. It was reported that meninges-derived fibroblasts strongly express axonal growth–inhibitory molecules, such as semaphorin 3A.30,36 Actually, the present study showed that fibronectin-positive cells were present in the lesion site of the vehicle-treated animals (Fig. 3) and they may have been able to repulse the regenerating axons. Histological analysis demonstrated that the newly generated tissues were predominantly composed of FIFs that filled the large cystic cavities around the lesion site. Surprisingly, numerous axons coursed through these tissues, suggesting that the FIFs played a key role in nerve regeneration. From these observations, we concluded that the FIFs had characteristics different from those of meningeal cells. Therefore, we focused on analyzing the cell properties of the FIFs to understand the mechanisms underlying this functional recovery.
The cultured FIFs strongly expressed protein and mRNA of N‐cadherin, a calcium-dependent cell-adhesion molecule that promotes axonal initiation and extension,31,32 but the MDFs used as a control did not (Figs. 6 and 8). The ratio of cortical neurons with longer neurites was higher when the neurons were seeded on FIF monolayers than on the MDF monolayers (Fig. 7). These observations suggest that the FIFs promoted neuritegenesis, partly via interactions of N‐cadherin on their membranes with neurites. Moreover, the mRNA levels of neurotrophic factors such as NGF and BDNF were higher in the FIFs than in the MDFs (Fig. 8). The enhanced expression of these neurotrophic factors may be favorable for neuritegenesis, because the delivery of neurotrophic factors has been reported to enhance the regeneration of spinal motor pathways such as the corticospinal tract and RCT.11,37,38 Thus, as we thought that FIFs play a key role in axonal regeneration in FGF-2-treated SCI, we transplanted the FIFs into the lesion site. The animals transplanted with the FIFs attained BBB scores exceeding 7, as expected, indicating that the animals could extensively move two hindlimb joints, and somewhat move the third one (Fig. 9). Many GAP-43-immnoreactive axons of the host crossed the transplanted FIFs, which survived well in the host tissue (Fig. 11). As GAP-43 mRNA expression is one of the markers of neurons regenerating axons, as was shown in the case of axotomized magnocellular rubrospinal neurons,39 these results strongly support the idea that the FIFs enhance locomotor recovery after SCI via formation of a permissive microenvironment for axonal regrowth. We believe that the axonal regrowth of the RST shown in Figure 10 contributed to this improvement, because this tract is predominantly involved in rodent locomotor function.40,41
The present study is a unique demonstration of successful cell therapy for axonal regeneration after complete SCI. In addition, we have established a reproducible method for the preparation of mass cultures of these FIFs by utilizing the proliferation induced by FGF-2. One of the striking properties of FIFs is the stability of their ability to promote axonal regeneration, because they could ameliorate locomotor dysfunction even after having proliferated over several passages (Kasai et al., unpublished results), implying that cell therapy using FIFs would have the advantages of high efficacy and good reproducibility. Mesenchymal stem cells (MSCs) are expected to be most useful in regeneration of various tissues, including those of the nervous system, because they can be expanded in culture and reintroduced into patients as autografts or allografts. For instance, MSCs from bone marrow significantly improve the locomotor function of contused SCI rats.42,43 We have evidence that FIFs constitutively express the genes of CD90 (THY1), CD105 (ENG), and CD106 (VCAM1), which were previously shown to be mesenchymal markers,44,45 as well as markers of endothelial cells, such as VE-cadherin, thrombomodulin, and VEGFR-1, demonstrating that FIFs have many characteristics in common with other MSCs, such as bone marrow-derived MSCs. These observations also suggest the possibility that the FIFs are extrinsic cells derived from tissues other than the spinal cord. However, our preliminary results showed that FIFs are resident cells in the spinal cord, because they could be cultured from uninjured spinal cord explants. Moreover, FGF-2-induced fibronectin-positive cells obtained from the bone marrow, blood, or skin did not improve locomotor function when transplanted into the injury site (Kasai et al., unpublished results). The expression of cell markers was not completely identical between the FIFs and the FGF-2-responsive fibronectin-positive cells of the bone marrow. For instance, VEGFR-2 was expressed in the bone marrow–derived cells, but not in the FIFs, and the situation was reversed for CD34 antigen. Therefore we suppose that the FIFs are probably a part of the population of spinal cord–resident MSCs that has unique properties specific to the spinal cord to afford marked stimulation of nerve regeneration. It is possible that FIFs transplanted into the injured spinal cord survive well even without immunosuppressants, and their survival is likely due to immunological tolerance, also a characteristic of MSCs.46
Conclusions
Based on the results of the first half of our study, we concluded that the FGF-2-enhanced recovery of hindlimb locomotor function was mediated by the massive induction of FIFs that provided an environment that promotes axonal growth. This conclusion is supported by the findings of the second part of the study, which showed that the transplantation of FIFs into the lesion site dramatically improved locomotor function to a degree that has never before been reported. Due to the high efficacy of the repair, the use of these FIFs in human SCI patients is quite promising, and is anticipated to become possible in the near future. It may be most effective to utilize FIFs for the treatment of SCI in combination with other therapies, such as treatment with inhibitory compounds such as antibodies against Nogo-A,6 and semaphorin 3A inhibitor,47 or other types of immunotherapy such as the use of preactivated autologous macrophages48 or T-cell-based vaccinations.49 However, there are still many problems to address regarding the cellular characteristics of FIFs, particularly human ones, including their cell origin and lineage, and the efficacy of FGF-2 treatment and/or FIF transplantation in a contusion model of SCI. These questions will need to be answered before they can be used for clinical treatment of human SCI.
Acknowledgments
We thank Drs. K. Nishikawa and Y. Yoshitake (Kanazawa Medical University, Ishikawa Prefecture, Japan) for providing the FGF-2-producing E. coli cells, and Dr. W. Stallcup (Burnham Institute, La Jolla, CA) for the anti-NG2 antibody.
Author Disclosure Statement
This work was supported in part by a grant‐in‐aid for basic scientific research from the Ministry of Education, Science, and Culture of Japan, and by a grant for brain medical science from Gifu Prefecture.
References
- 1.Nathan P.W. (1994). Effects on movement of surgical incisions into the human spinal cord. Brain 117 (Pt. 2), 337–346 [DOI] [PubMed] [Google Scholar]
- 2.Iannotti C., Zhang Y.P., Shields L.B., Han Y., Burke D.A., Xu X.M., and Shields C.B. (2006). Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J. Neurotrauma 23, 853–865 [DOI] [PubMed] [Google Scholar]
- 3.Fitch M.T., and Silver J. (2008). CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yiu G., and He Z. (2006). Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Z., and Koprivica V. (2004). The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 27, 341–368 [DOI] [PubMed] [Google Scholar]
- 6.Schwab M.E. (2004). Nogo and axon regeneration. Curr. Opin. Neurobiol. 14, 118–124 [DOI] [PubMed] [Google Scholar]
- 7.Bulsara K.R., Iskandar B.J., Villavicencio A.T., and Skene J.H. (2002). A new millennium for spinal cord regeneration: growth‐associated genes. Spine 27, 1946–1949 [DOI] [PubMed] [Google Scholar]
- 8.Harel N.Y., and Strittmatter S.M. (2006). Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci. 7, 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto M., Nitta A., Fukumitsu H., Nomoto H., Shen L., and Furukawa S. (2005a). Inflammation‐induced GDNF improves locomotor function after spinal cord injury. Neuroreport 16, 99–102 [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M., Nitta A., Fukumitsu H., Nomoto H., Shen L., and Furukawa S. (2005b). Involvement of glial cell line‐ derived neurotrophic factor in activation processes of rodent macrophages. J. Neurosci. Res. 79, 476–487 [DOI] [PubMed] [Google Scholar]
- 11.Schnell L., Schneider R., Kolbeck R., Barde Y.A., and Schwab M.E. (1994). Neurotrophin‐3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 367, 170–173 [DOI] [PubMed] [Google Scholar]
- 12.Widenfalk J., Lundstromer K., Jubran M., Brene S., and Olson L. (2001). Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 21, 3457–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Presta. M, Dell'Era P., Mitola S., Moroni E., Ronca R., and Rusnati M. (2005). Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16, 159–178 [DOI] [PubMed] [Google Scholar]
- 14.Greenberg D.A., and Jin K. (2005). From angiogenesis to neuropathology. Nature 438, 954–959 [DOI] [PubMed] [Google Scholar]
- 15.Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., and Nakafuku M. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429–441 [DOI] [PubMed] [Google Scholar]
- 16.Ohori Y., Yamamoto S., Nagao M., Sugimori M., Yamamoto N., Nakamura K., and Nakafuku M. (2006). Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J. Neurosci. 26, 11948–11960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura S., Teramoto T., Whalen M.J., Irizarry M.C., Takagi Y., Qiu J., Harada J., Waeber C., Breakefield X.O., and Moskowitz M.A. (2003). FGF‐2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J. Clin. Invest. 112, 1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe K., and Saito H. (2001). Effects of basic fibroblast growth factor on central nervous system functions. Pharmacol. Res. 43, 307–312 [DOI] [PubMed] [Google Scholar]
- 19.Bansal R. (2002). Fibroblast growth factors and their receptors in oligodendrocyte development: implications for demyelination and remyelination. Dev. Neurosci. 24, 35–46 [DOI] [PubMed] [Google Scholar]
- 20.Rabchevsky A.G., Fugaccia I., Fletcher‐Turner A., Blades D.A., Mattson M.P., and Scheff S.W. (1999). Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J. Neurotrauma 16, 817–830 [DOI] [PubMed] [Google Scholar]
- 21.Rabchevsky A.G., Fugaccia I., Turner A.F., Blades D.A., Mattson M.P., and Scheff S.W. (2000). Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp. Neurol. 164, 280–291 [DOI] [PubMed] [Google Scholar]
- 22.Ibarra A., Hernández E., Lomeli J., Pineda D., Buenrostro M., Martiñón S., Garcia E., Flores N., Guizar‐Sahagun G., Correa D., and Madrazo I. (2007). Cyclosporin‐A enhances non‐ functional axonal growing after complete spinal cord transection. Brain Res. 1149, 200–209 [DOI] [PubMed] [Google Scholar]
- 23.Gómez‐Pinilla F, Vu L, and Cotman C.W. (1995). Regulation of astrocyte proliferation by FGF‐2 and heparan sulfate in vivo. J. Neurosci. 15, 2021–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walz A., McFarlane S, Brickman Y.G., Nurcombe V., Bartlett P.F., and Holt C.E. (1997). Essential role of heparan sulfates in axon navigation and targeting in the developing visual system. Development 124, 2421–2430 [DOI] [PubMed] [Google Scholar]
- 25.Squires C.H., Childs J., Eisenberg S.P., Polverini P.J., and Sommer A. (1988). Production and characterization of human basic fibroblast growth factor from Escherichia coli. J. Biol. Chem. 263, 16297–16302 [PubMed] [Google Scholar]
- 26.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor 7. rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 27.Nitta A., Ito M., Fukumitsu H., Ohmiya M., Ito H., Sometani A., Nomoto H., Furukawa Y., and Furukawa S. (1999). 4‐Methylcatechol increases brain‐derived neurotrophic factor content and mRNA expression in cultured brain cells and in rat brain in vivo. J. Pharmacol. Exp. Ther. 291, 1276–1283 [PubMed] [Google Scholar]
- 28.Ito H., Nakajima A., Nomoto H., and Furukawa S. (2003). Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix‐loop‐helix transcription factors Mash1 and Math1. J. Neurosci. Res. 71, 648–658 [DOI] [PubMed] [Google Scholar]
- 29.Furukawa S., and Furukawa Y. (2007). FGF‐2‐treatment improves locomotor function via axonal regeneration in the transected rat spinal cord. Brain Nerve 59, 1333–1339 [PubMed] [Google Scholar]
- 30.Niclou S.P., Franssen E.H., Ehlert E.M., Taniguchi M., and Verhaagen J. (2003). Meningeal cell‐derived semaphorin 3A inhibits neurite outgrowth. Mol. Cell Neurosci. 24, 902–912 [DOI] [PubMed] [Google Scholar]
- 31.Riehl R., Johnson K., Bradley R., Grunwald G.B., Cornel E., Lilienbaum A., and Holt C.E. (1996). Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron 17, 837–848 [DOI] [PubMed] [Google Scholar]
- 32.Tomaselli K.J., Neugebauer K.M., Bixby J.L., Lilien J., and Reichardt L.F. (1988). N‐cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron 1, 33–43 [DOI] [PubMed] [Google Scholar]
- 33.Jimenez Hamann M.C., Tator C.H., and Shoichet M.S. (2005). Injectable intrathecal delivery system for localized administration of EGF and FGF‐2 to the injured rat spinal cord. Exp. Neurol. 194, 106–119 [DOI] [PubMed] [Google Scholar]
- 34.Kojima A., and Tator C.H. (2002). Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J. Neurotrauma 19, 223–238 [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez A.M., Carman L.S., Ong M., Ray J., Gage F.H., Shults C.W., and Baird A. (1994). Storage, metabolism, and processing of 125I‐fibroblast growth factor‐2 after intracerebral injection. Brain Res. 665, 285–292 [DOI] [PubMed] [Google Scholar]
- 36.De Winter F., Oudega M., Lankhorst A.J., Hamers F.P., Blits B., Ruitenberg M.J., Pasterkamp R.J., Gispen W.H., and Verhaagen J. (2002). Injury‐induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 175, 61–75 [DOI] [PubMed] [Google Scholar]
- 37.Grill R., Murai K., Blesch A., Gage F.H., and Tuszynski M.H. (1997). Cellular delivery of neurotrophin‐3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 17, 5560–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Kim D., Himes B.T., Chow S.Y., Schallert T., Murray M., Tessler A., and Fischer I. (1999). Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J. Neurosci. 19, 4370–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tetzlaff W., Alexander S.W., Miller F.D., and Bisby M.A. (1991). Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP‐43. J. Neurosci. 11, 2528–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuchler M., Fouad K., Weinmann O., Schwab M.E., and Raineteau O. (2002). Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J. Comp. Neurol. 448, 349–359 [DOI] [PubMed] [Google Scholar]
- 41.Muir G.D., and Whishaw I.Q. (2000). Red nucleus lesions impair overground locomotion in rats: a kinetic analysis. Eur. J. Neurosci. 12, 1113–1122 [DOI] [PubMed] [Google Scholar]
- 42.Cizkova D., Rosocha J., Vanicky I., Jergova S., and Cizek M. (2006). Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol. Neurobiol. 26, 1167–1180 [DOI] [PubMed] [Google Scholar]
- 43.Dasari V.R., Spomar D.G., Cady C., Gujrati M., Rao J.S., and Dinh D.H. (2007). Mesenchymal stem cells from rat bone marrow downregulate caspase‐3‐mediated apoptotic pathway after spinal cord injury in rats. Neurochem. Res. 32, 2080–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Ugarte D.A., Alfonso Z., Zuk P.A., Elbarbary A., Zhu M., Ashjian P., Benhaim P., Hedrick M.H., and Fraser J.K. (2003). Differential expression of stem cell mobilization‐associated molecules on multi‐lineage cells from adipose tissue and bone marrow. Immunol. Lett. 89, 267–270 [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki T., Kitagawa Y., Toriyama K., Kobori M., and Torii S. (2005). Isolation of two human fibroblastic cell populations with multiple but distinct potential of mesenchymal differentiation by ceiling culture of mature fat cells from subcutaneous adipose tissue. Differentiation 73, 69–78 [DOI] [PubMed] [Google Scholar]
- 46.Uccelli A., Moretta L., and Pistoia V. (2008). Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 [DOI] [PubMed] [Google Scholar]
- 47.Kaneko S., Iwanami A., Nakamura M., Kishino A., Kikuchi K., Shibata S., Okano H.J., Ikegami T., Moriya A., Konishi O., Nakayama C., Kumagai K., Kimura T., Sato Y., Goshima Y., Taniguchi M., Ito M., He Z., Toyama Y., and Okano H. (2006). A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 12, 1380–1389 [DOI] [PubMed] [Google Scholar]
- 48.Rapalino O., Lazarov‐Spiegler O., Agranov E., Velan G.J., Yoles E., Fraidakis M., Solomon A., Gepstein R., Katz A., Belkin M., Hadani M., and Schwartz M. (1998). Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat. Med. 4, 814–821 [DOI] [PubMed] [Google Scholar]
- 49.Moalem G., Leibowitz‐Amit R., Yoles E., Mor F., Cohen I.R., and Schwartz M. (1999). Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 5, 49–55 [DOI] [PubMed] [Google Scholar]