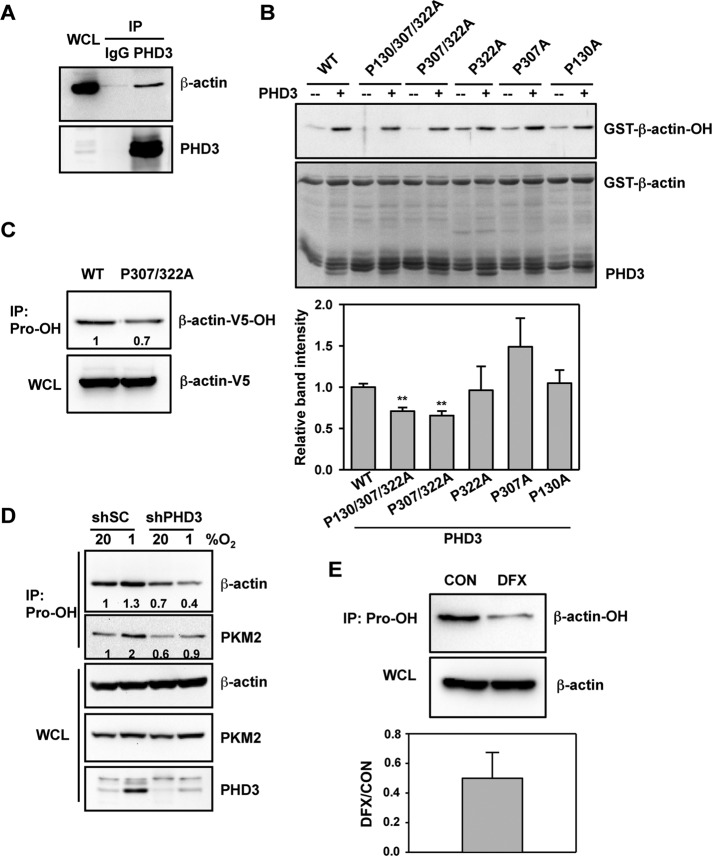
FIGURE 2:
PHD3 mediates hydroxylation of actin on Pro-307 and Pro-322. (A) HeLa cells were exposed to 1% O2 for 24 h. IP assays were performed using IgG or anti-PHD3 antibody, followed by immunoblot assays using antibodies against β-actin or PHD3. WCL, whole-cell lysate. (B) In vitro hydroxylation assays were performed using purified WT or mutant GST–β-actin and PHD3, followed by immunoblot assays with anti-hydroxyproline antibody. The immunoblot bands were quantified by densitometry and normalized to WT (mean ± SEM, n = 3). **p < 0.01 vs. WT. (C) HeLa cells were transfected with vector encoding WT β-actin-V5 or β-actin (P307/322A)-V5 and exposed to 1% O2 for 24 h. IP of WCLs was performed using anti-hydroxyproline antibody (Pro-OH), followed by immunoblot assays with anti-V5 antibody. The immunoblot bands were quantified by densitometry and normalized to WT. Representative blots from two independent experiments. (D) HeLa-shSC or HeLa-shPHD3 cells were exposed to 20% or 1% O2 for 24 h. IP of WCLs was performed using anti-hydroxyproline antibody (Pro-OH), followed by immunoblot assays with antibodies against the indicated proteins. The immunoblot bands were quantified by densitometry and normalized to shSC-20% O2. Representative blots from two independent experiments. (E) HeLa cells were treated with desferrioxamine (DFX, 100 μM) for 6 h. IP of WCLs was performed using anti-hydroxyproline antibody (Pro-OH), followed by immunoblot assays with anti–β-actin antibody. The immunoblot bands were quantified by densitometry and normalized to control (CON). Data shown are mean ± SEM, n = 3.