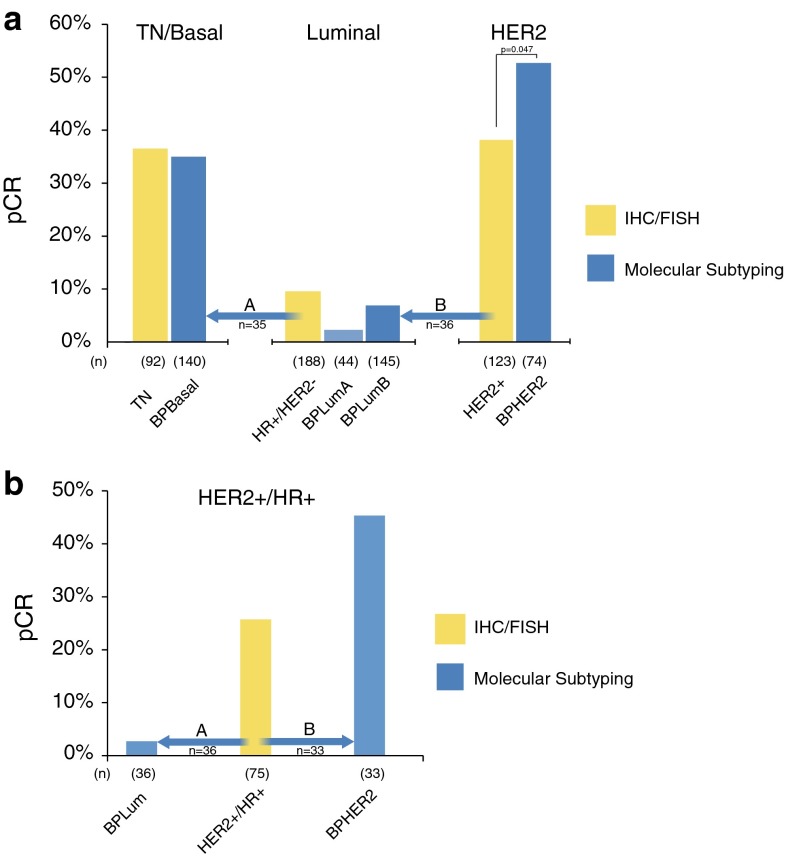
Fig. 1.
a pCR rates and major subtype re-assignments for patients classified by BluePrint/MammaPrint molecular subtyping compared with IHC/FISH assessed subgroups. The analysis includes only patients treated with NCT (n = 403). The two major subtype reassignments were (A) conventional luminal (HR+/HER2−) patients, 35 of 188 (19 %) patients reclassified by BluePrint as Basal (arrow A) and (B) conventional HER2+ patients, 36 of 123 (29 %) reclassified by BluePrint as Luminal (arrow B). b pCR rates and major subtype reassignments for conventional HER2+/HR+ (“triple positive”) patients (95 % treated with NCT/trastuzumab). Thirty-six of 75 (48 %) of conventional HER2+/HR+ patients were reclassified by BluePrint as Luminal—with only 1 pCR (3 %) to NCT (arrow A). Thirty-three of 75 (44 %) of conventional HER2+/HR+ patients were classified by BluePrint as HER2, with a pCR rate to NCT of 45 % (arrow B). Six conventional HER2+/HR+ patients were reassigned to BPBasal (not shown)