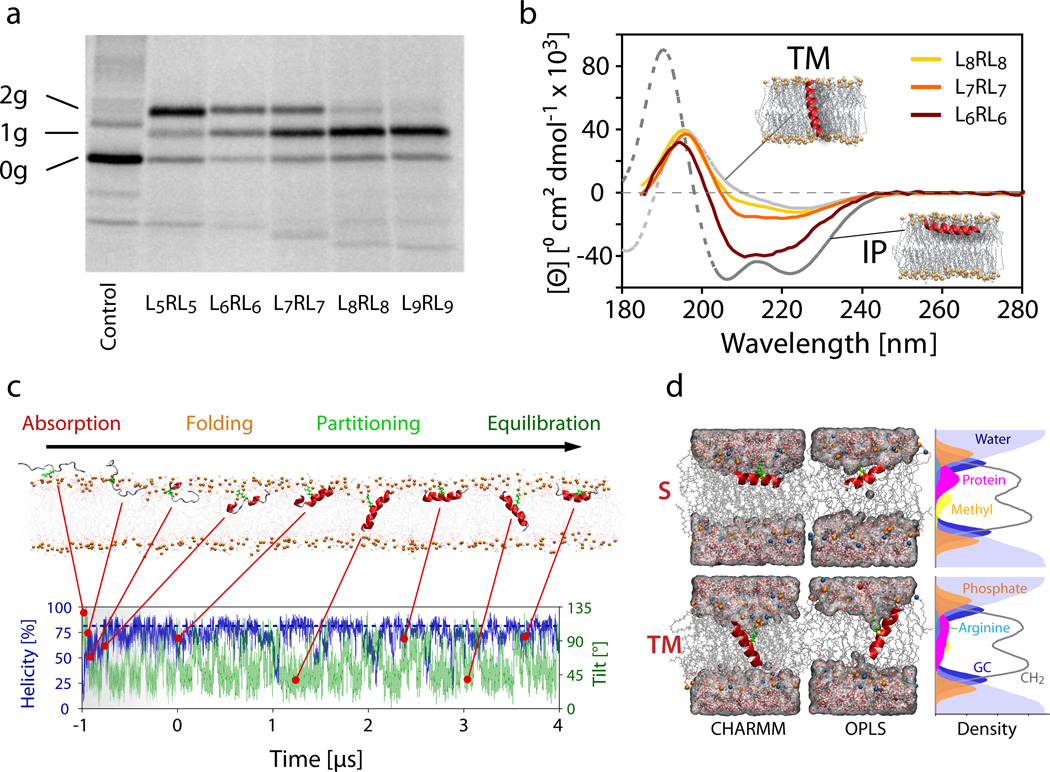
Figure 2.
Experimental data for in vitro translocon-guided insertion, orientation of synthetic peptides in POPC lipid bilayers, and simulations of spontaneous insertion. (a) SDS-PAGE analysis of LepB constructs containing the GGPG-LnRLn-GPGG peptides as an added H-segment. Glycosylation sites on either side of the segment allow quantification of inserted (singly glycosylated, 1g) and non-inserted (doubly glycosylated, 2g) configurations. Increasing the number of leucine residues in the H-segment shifts the equilibrium from doubly to singly glycosylated peptides, with L6RL6 and L7RL7 showing substantial populations of both forms. Proteins that remain unglycosylated (0g), never entered the translocon and are excluded from the measurement. The full SDS-PAGE gel is presented in Supplementary Figure 1. (b) Oriented synchrotron radiation circular dichroism (O-SRCD) spectra of the G-WLn-1RLn-G peptides in stacked POPC lipid bilayers (peptide/lipid = 1/100, relative hydration = 100%). Theoretical reference spectra31,32 for α-helical peptides oriented parallel (TM, tilt angle ≈ 0°) and perpendicular (IP, tilt angle ≈ 90°) to the membrane normal (coaxial to the light beam) are shown in grey. (c) Unbiased folding-partitioning simulation of the G-L7RL7-G (L7RL7) peptide (c.f. Table 1). Absorption and interfacial folding is rapid and typically completed within 400 ns. The arginine residue, which is highlighted in green, retains contact with the lipid head group phosphates at all times (see Supplementary Table 1). (d) Representative snapshots of the L7RL7 peptide simulation showing the two dominant states present at equilibrium. The configurations consist of a surface (S) adsorbed and a transmembrane (TM) inserted helical peptide, irrespective of the force field employed. The density profiles are averages over the S and TM states of the OPLS simulation of L7RL7 (tS = 70 ns, tTM = 130 ns). In the TM state, the arginine residue is buried more deeply in the hydrophobic region of the bilayer. Phosphates (orange spheres) and water are frequently pulled into the bilayer to help solvate the charged guanidinium group. The Gaussian distribution of the peptide on the bilayer leaflet is noticeably broader and reaches deeper into the hydrophobic bilayer core.