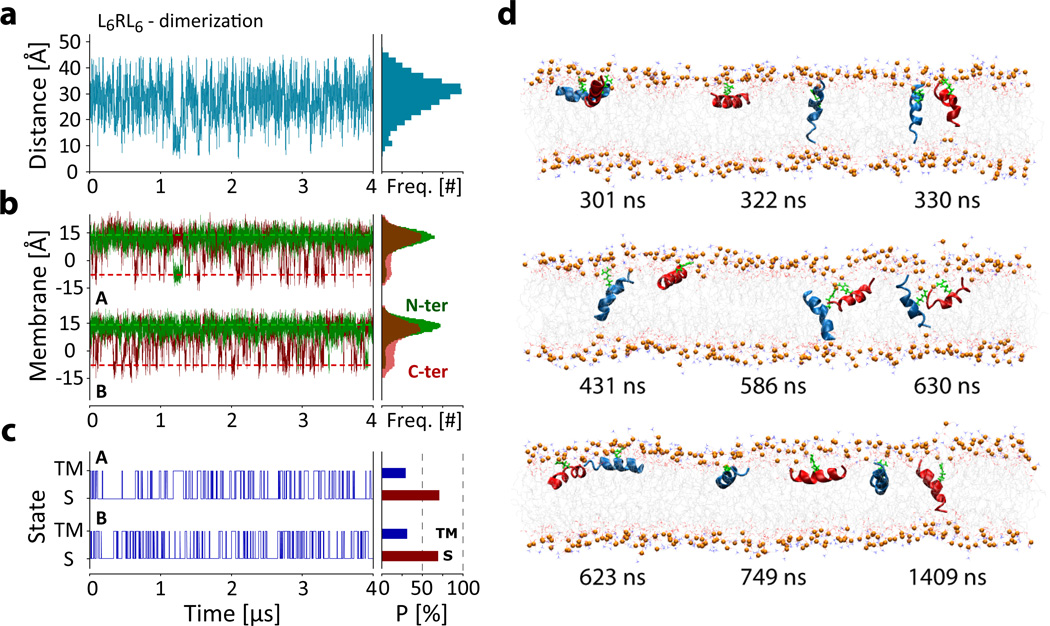
Figure 5.
Equilibrium simulation (4 µsec) of two L6RL6 peptides to test for dimerization. (a) Analysis of the inter-arginine distance. The figure plots the distance between the CZ atoms of the two guanidinium sidechains. The figure shows that the peptides diffuse randomly, independent of each other and any aggregate formation is short-lived, with the longest observed contact lasting ~90 ns (at 1.17 µs). (b) Analysis of the positions of the peptide N-termini (green) and C-termini (red) along the membrane normal reveals that each peptide partitions independently of the other. (c) The state transitions from the analysis of the peptide termini position along the membrane normal (panel B) show that both peptides have similar S and TM state populations and associated free energies (see Table 1). (d) Snapshots of the dimer simulation show independent partitioning of the peptides with occasional random collision, no stable aggregate formation was observed. See movies LnRLn_dimer_side.mov and LnRLn_dimer_top.mov in online Supplementary Information.