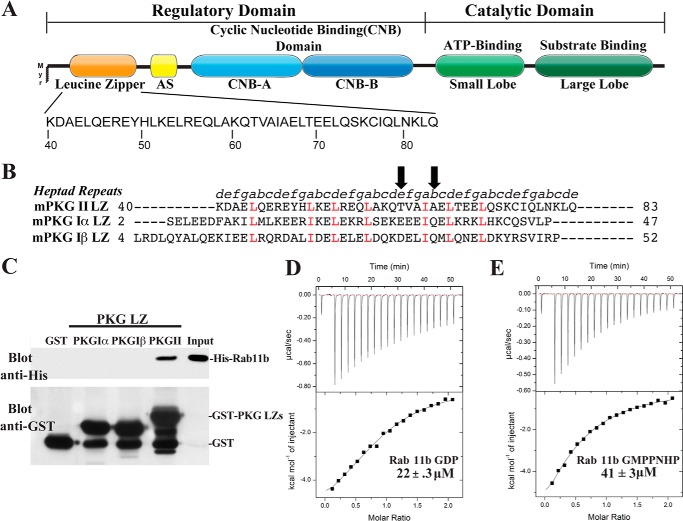
FIGURE 1.
Domain organization and direct binding of PKG II LZ and Rab11b. A, domain organization of PKG II with the sequence of the leucine zipper domain highlighted. AS, autoinhibitory sequence; Myr, myristoylation site. B, sequence alignment of the PKG Iα, PKG Iβ, and PKG II LZ domains. Positions within the heptad repeat (a–g) are shown at the top. The key interface residues, Thr-62 and Ala-66, mutated for disrupting the PKG II LZ and Rab11b interaction are marked with arrows. C, GST or GST-fusion proteins containing the PKG LZs were immobilized on glutathione-Sepharose beads and incubated with purified His-tagged Rab11b as described under “Experimental Procedures.” Beads were washed, and bound proteins were analyzed by SDS-PAGE/immunoblotting. Blots were probed with an antibody specific for the His tag to detect Rab11b binding and GST to demonstrate equal loading of GST and the GST-tagged PKG leucine zippers. D and E, isothermal titration calorimetry experiments demonstrate that PKG II LZ (residues 40–83) has a slightly higher binding preference for Rab11b-GDP (D) than Rab11b bound to the nonhydrolyzable GTP analog GMPPNHP (E).