FIGURE 3.
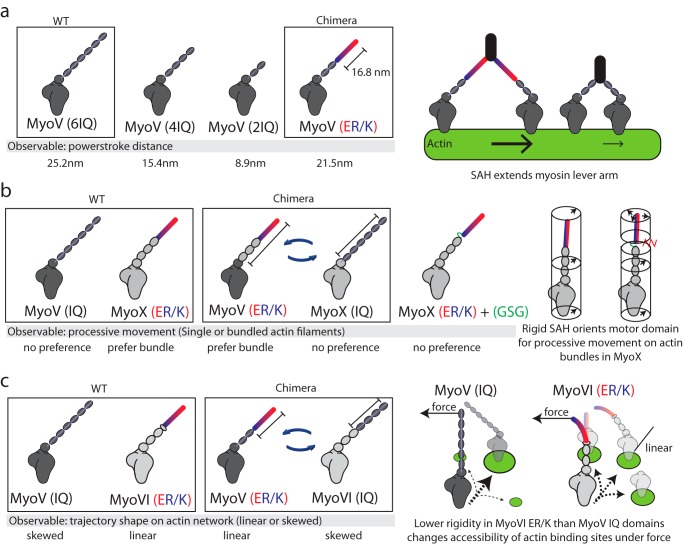
The ER/K α-helix is a modular genetic motif that can be used to create myosin chimeras with altered mechanical properties. a, Baboolal et al. (33) created a myosin V (MyoV) chimera containing the putative ER/K α-helix from Dictyostelium myosin M. Left, the power stroke distances of WT myosin V, myosin V with truncation of two or four calmodulin stabilized IQ domains, and a chimera of myosin V with two native IQ domains and a 16.8-nm ER/K α-helix. Right, an extended and rigid ER/K α-helix can propagate force generated in the myosin catalytic domain to facilitate long processive steps on actin filaments. b, Nagy and Rock (34) generated multiple chimeras between myosin V and myosin X (MyoX) to assess structural elements that allow myosin X to preferably move on fascin-actin bundles. Left, representative chimeras that were used to identify that in myosin X, the ER/K α-helix and not the motor domain or step size dictates processive movement on fascin-actin bundles. Right, insertion of unstructured Gly-Ser-Gly residues between the SAH and IQ domains of myosin X disrupts preferential processivity on fascin-actin bundles. The ER/K α-helix alters the orientation of the motor domain, allowing it to favorably bind actin sites uniquely presented in fascin-actin bundles. c, Hariadi et al. (35) generated chimeras swapping the ER/K α-helix from myosin VI (MyoVI) with the IQ domains from myosin V while investigating the collective movement of multiple myosins tethered together. Left, multiple myosin V proteins, but not myosin VI, display meandering trajectories while traversing actin meshworks. Swapping regions of the lever arm containing the ER/K α-helix can reverse this phenomenon. Right, the IQ domains are likely more rigid than the ER/K α-helix, such that the inter-myosin force can selectively alter the accessibility of actin binding sites for the less rigid myosin VI.