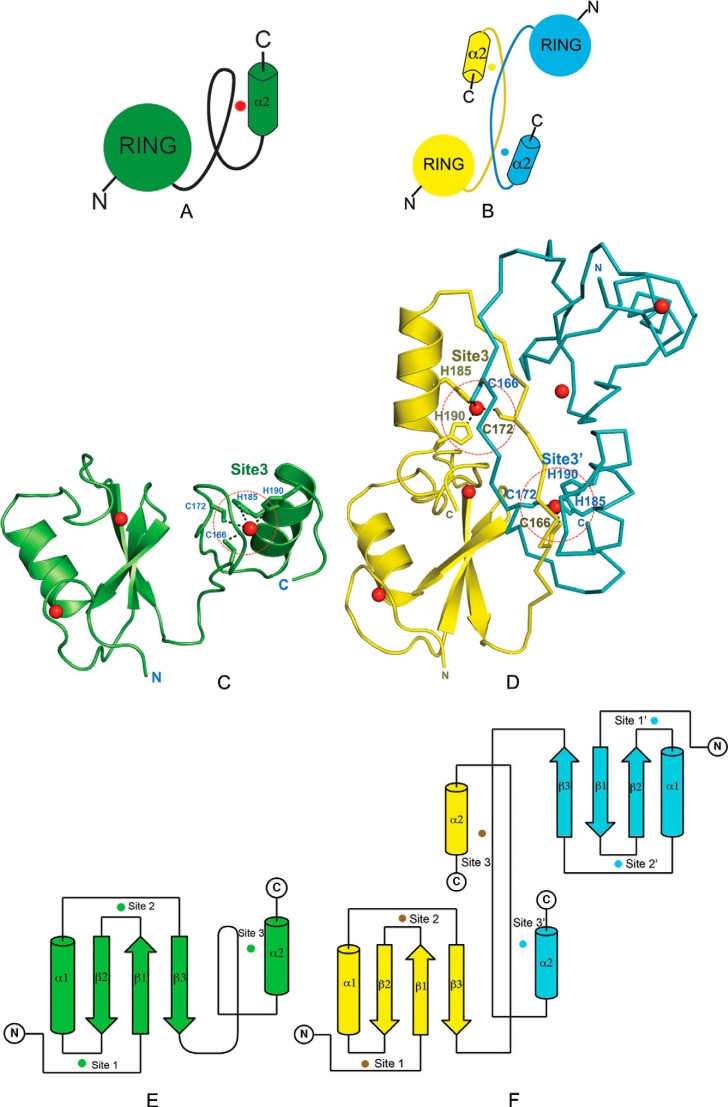
FIGURE 4.
C2H2 ZnF in the C-terminal domain of HYBΔC is critical for its monomeric fold. Analysis of the structural differences between HYBΔC and HYB (A) and the dimeric fold of HYB domain containing paired Hakai (aa 106–206) (PDB code 3vk6) monomers (B). The red dot in A represents the zinc ion in the C-terminal domain of HYBΔC, whereas the cyan and yellow dots in B represent the C-terminal zinc ion shared between the respective monomers of the dimeric HYB-fold. C, the monomeric fold of HYBΔC (green) contains a C2H2 ZnF in the C-terminal domain (highlighted by red dotted circle). D, the HYB domain (PDB code 3vk6) contains a dimeric fold of paired Hakai (aa 106–206) monomers (yellow monomer in ribbon representation; cyan in schematic representation for clarity). In monomeric HYBΔC, all four zinc-coordinating residues are situated in the same monomeric chain. Comparatively, the dimeric fold of the HYB domain is formed by the sharing of two zinc ions (red spheres) between the Hakai monomers, such that three of the four zinc-binding residues come from one monomer (His185, His190, and Cys172) and the fourth from the adjacent monomer (Cys166). The zinc-coordinating side chains are shown as sticks. The structural rearrangement arises because of a systematic reshuffling among the zinc-coordinating residues in the monomeric and dimeric conformers. Topology of HYBΔC (E) is compared with that of the HYB domain (PDB code 3vk6) (F). The zinc ions are shown as spheres (color coded according to the monomer colors) and the three zinc-coordination sites are indicated for each monomer (Sites 1, 2, and 3). In HYBΔC, Site 3 is formed by four zinc-coordination residues, all situated from the same monomer. For the HYB domain, Site 3 and Site 3′ share residues from both monomers.