FIGURE 8.
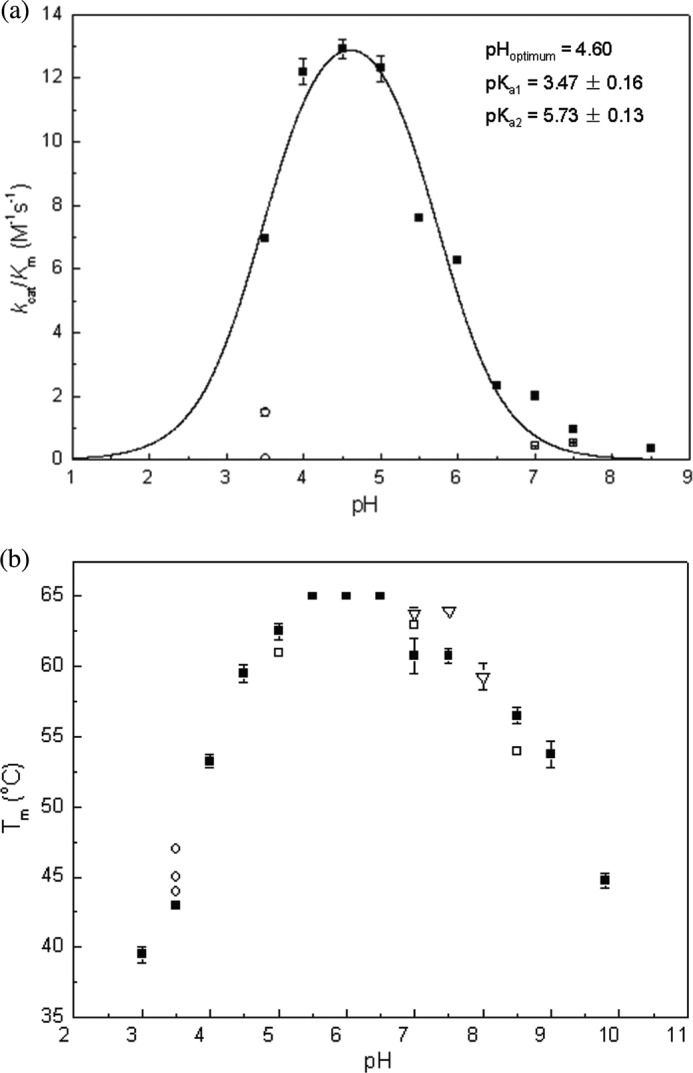
pH dependence of kcat/Km (a) and melting temperature Endo H (b) of FgFCO1. All steady-state reactions were performed with pNP-Fuc at 37 °C. The kinetic data (filled squares) were fit to kcat/Km = (kcat/Km)max/(1 + 10pKa1 − pH + 10pH − pKa2). Error bars indicate S.D. a, ■, 50 mm buffers of sodium citrate, pH 3, sodium acetate (pH 3.5–5.5), MES (pH 6–6.5), HEPES (pH 7–7.5), and Bicine (pH 8.5); □, 50 mm Tris at pH 7 and pH 7.5, respectively; ○, 10 mm citrate and 40 mm sodium acetate (upper circle), 50 mm citrate (lower circle) at pH 3.5. b, ■, the same as in a; □, the same buffer system as ■ plus 100 mm NaCl; ○, 1 mm citrate and 50 mm sodium acetate (lower), 10 mm citrate and 40 mm sodium acetate (middle), 50 mm citrate (upper) at pH 3.5; ▿, 50 mm Tris (pH 7–8).
