FIGURE 9.
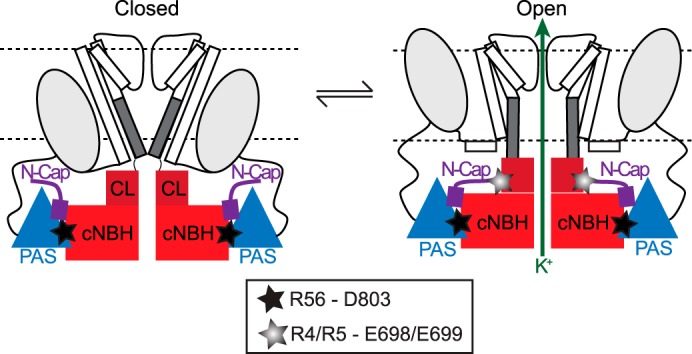
Proposed molecular mechanism for slow deactivation in Kv11. 1 channels. Cartoon of two opposing subunits of a tetrameric Kv11.1 channel shown in the closed (left) and open (right) conformations. In both conformations, Arg56 in the PAS domain (blue triangles) and Asp803 in the cNBH domain (red squares) form a highly stable interaction (denoted by black star). In the open conformation, Arg4 and Arg5 in the N-Cap domain (shown in purple) and Glu698 and Glu699 in the C-linker (shown in maroon) form an additional, but more transient, interaction (denoted by gray star) that stabilizes the open state, presumably by stabilizing the S6 activation gate (represented by dark gray bar) in the open conformation. The stable PAS-cNBH domain interaction is critical because it positions the N-Cap domain in the correct location to interact with the C-linker. These interactions underlie the slow deactivation gating observed in Kv11.1 channels.
