Abstract
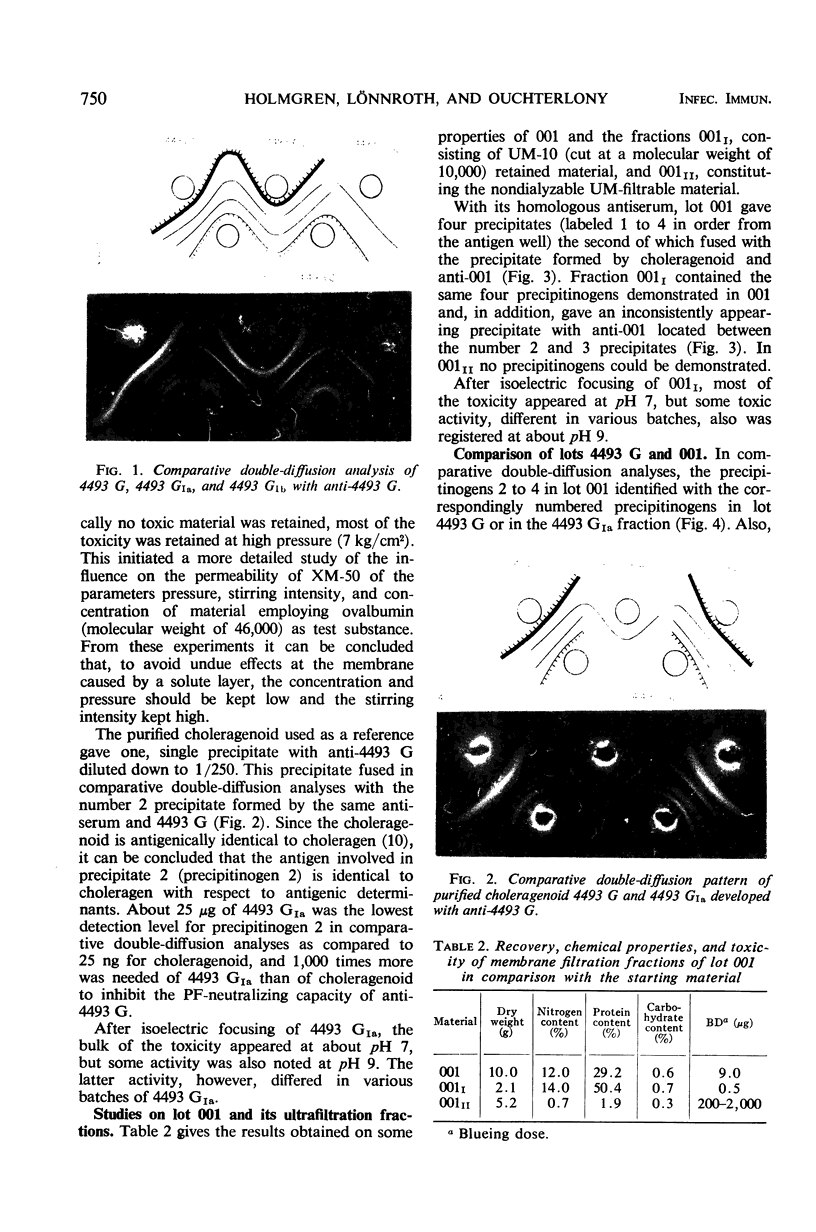
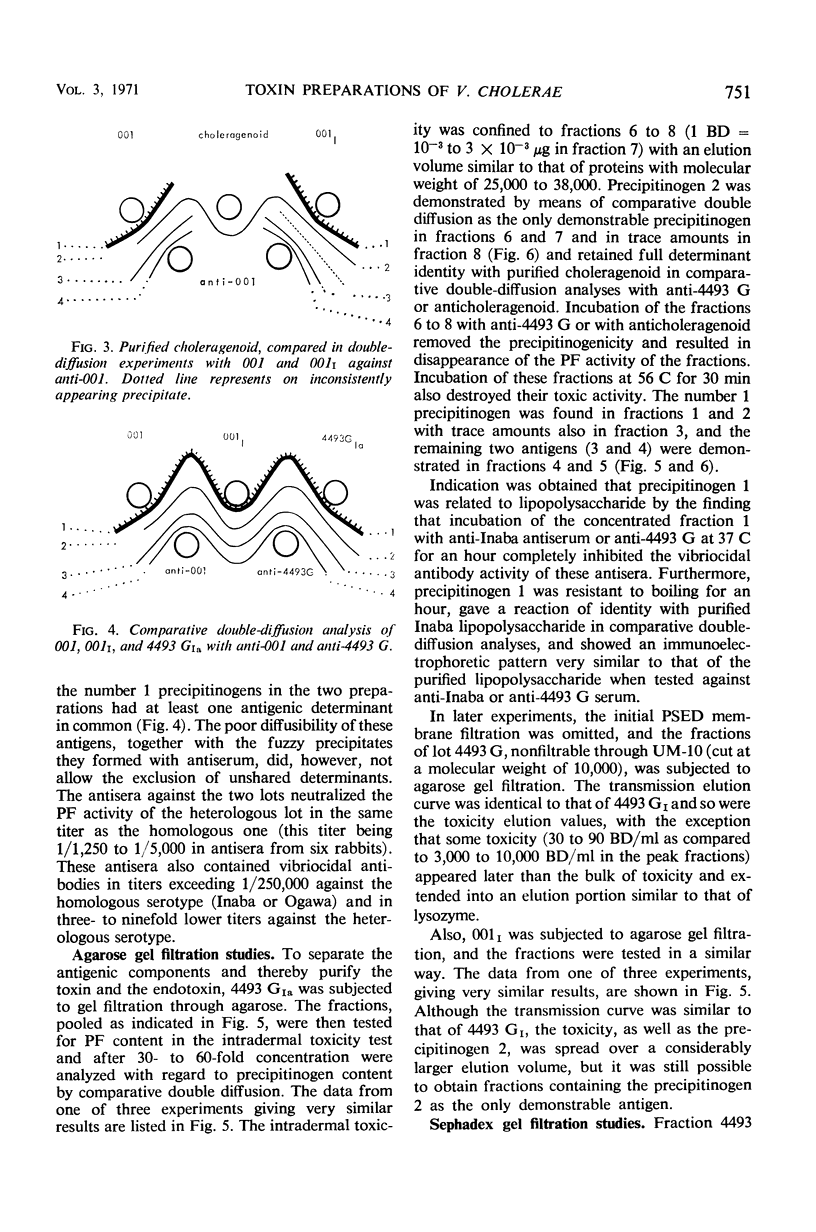
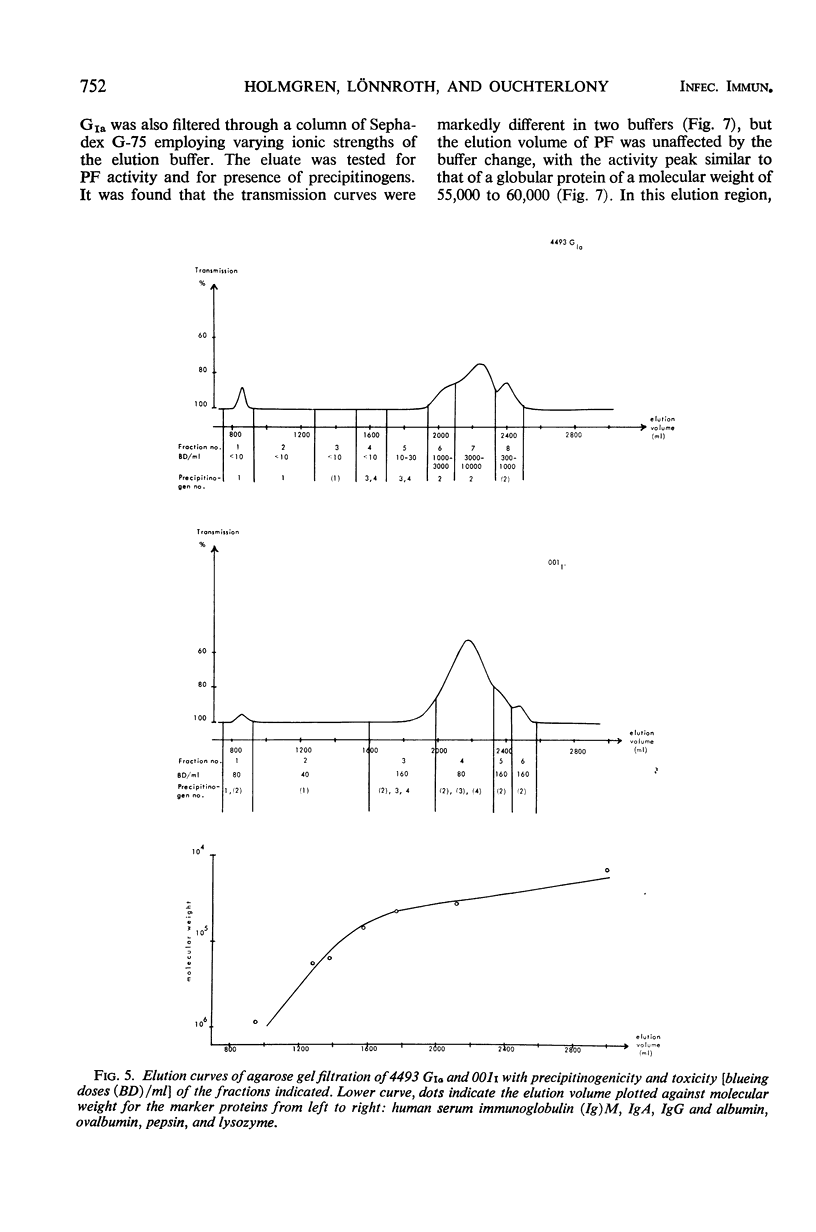
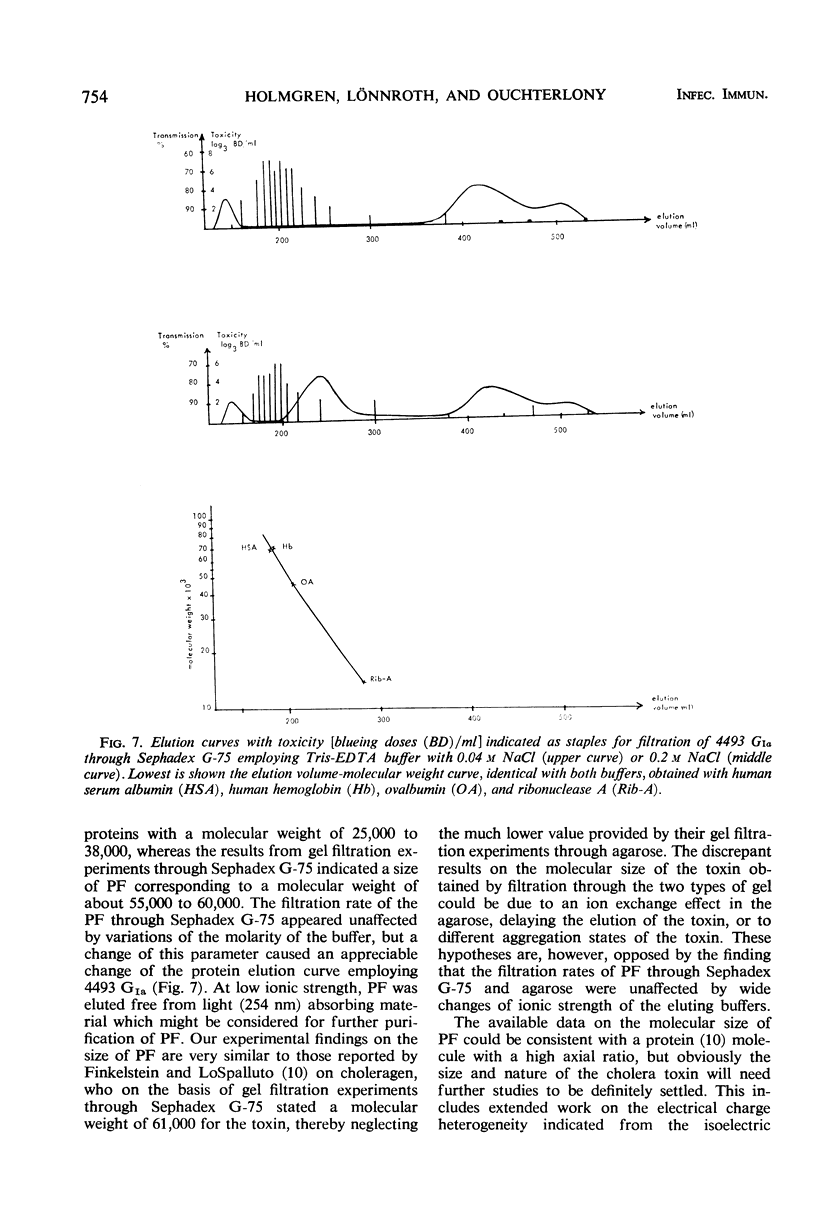
Two crude toxin preparations of Vibrio cholerae, labeled lot 4493 G (Inaba) and lot 001 (Ogawa), consisting of freeze-dried culture filtrate, were studied with regard to toxicity, precipitinogenicity, and chemical composition. Lot 4493 G contained much more carbohydrate and less protein than lot 001, but both of the preparations contained three precipitinogens which were identical. One of these factors was identified as cholera exotoxin. In addition, both contained a fourth cross-reacting precipitinogen related to lipopolysaccharide. The toxicity of the lots was very similar. The toxin active as permeability factor in the intradermal test, identified completely in comparative double-diffusion analyses with the toxin causing cholera-like symptoms in experimental animals, strongly indicated that the same toxin molecule was active in the two different model systems. The isoelectric point of the toxin was about pH 7, but some toxic activity focused toward a pH of 9. The toxicity was completely retained by a UM-10 membrane (cut at a molecular weight of 10,000), practically retained by a PSED membrane (cut at a molecular weight of 25,000), but not retained by an XM-50 membrane (cut at a molecular weight of 50,000). By gel filtration through agarose, it was possible to separate and purify more than 1,000-fold the toxin from the other, more rapidly filtering three precipitinogens. With lot 4493 G, the toxicity of the agarose gel filtration fractions was restricted to an elution volume similar to that of globular proteins with a molecular weight of 25,000 to 38,000, whereas gel filtration experiments through Sephadex G-75 indicated a size of the toxin corresponding to a molecular weight of about 55,000 to 60,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benenson A. S., Saad A., Mosley W. H., Ahmed A. Serological studies in cholera. 3. Serum toxin neutralization--rise in titre in response to infection with Vibrio cholerae, and the level in the "normal" population of East Pakistan. Bull World Health Organ. 1968;38(2):287–295. [PMC free article] [PubMed] [Google Scholar]
- Burrows W. Cholera toxins. Annu Rev Microbiol. 1968;22:245–268. doi: 10.1146/annurev.mi.22.100168.001333. [DOI] [PubMed] [Google Scholar]
- Coleman W. H., Kaur J., Iwert M. E., Kasai G. J., Burrows W. Cholera toxins: purification and preliminary characterization of ileal loop reactive type 2 toxin. J Bacteriol. 1968 Oct;96(4):1137–1143. doi: 10.1128/jb.96.4.1137-1143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Craig J. P. Preparation of the vascular permeability factor of Vibrio cholerae. J Bacteriol. 1966 Sep;92(3):793–795. doi: 10.1128/jb.92.3.793-795.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlin G. T., Craig J. P., Subong A., Carpenter C. C. Antitoxic immunity in experimental canine cholera. J Infect Dis. 1970 May;121(5):463–470. doi: 10.1093/infdis/121.5.463. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., PANSE M. V., KULKARNI D. R. Role of cholera a toxin in experimental cholera. J Bacteriol. 1959 Oct;78:594–595. doi: 10.1128/jb.78.4.594-595.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Production of highly purified choleragen and choleragenoid. J Infect Dis. 1970 May;121(Suppl):63+–63+. doi: 10.1093/infdis/121.supplement.s63. [DOI] [PubMed] [Google Scholar]
- Jacobs S. The determination of nitrogen in biological materials. Methods Biochem Anal. 1965;13:241–263. doi: 10.1002/9780470110317.ch5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nilsson L. A. Comparative testing of precipitation methods for quantitation of C-reactive protein in blood serum. Acta Pathol Microbiol Scand. 1968;73(1):129–144. doi: 10.1111/j.1699-0463.1968.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C., Steenburg R. W., Pierce N. F. Experimental cholera. A canine model. Lancet. 1966 Jul 23;2(7456):206–207. doi: 10.1016/s0140-6736(66)92484-6. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C. A slide microtechnique for the analysis of immune precipitates in gel. Int Arch Allergy Appl Immunol. 1957;10(6):355–360. doi: 10.1159/000228394. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C., HANSON L. A. Comparative analysis of immune electrophoretic precipitates employing a modified immune electrophoretic technique. Int Arch Allergy Appl Immunol. 1960;17:165–177. doi: 10.1159/000229122. [DOI] [PubMed] [Google Scholar]