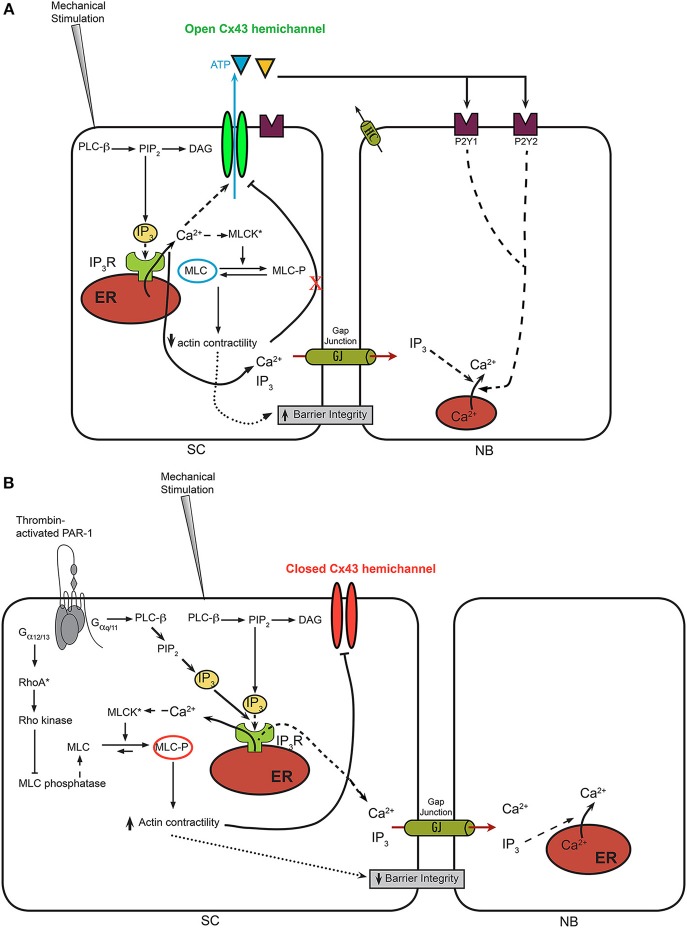
Figure 2.
A model for Ca2+-wave propagation in BCECs in normal conditions vs. thrombin-treated conditions. (A) In normal BCECs, it is hypothesized that mechanical stimulation leads to a moderate rise in cytosolic [Ca2+] via IP3-dependent signaling mechanisms, which leads to the opening of Cx43 hemichannels and the flux of ATP from the cytosol into the extracellular environment. This allows the propagation of the Ca2+ from the “stimulated cell” (SC) to neighboring (NB) cells via activation of purinergic receptor and downstream IP3-induced Ca2+ signaling. Under these conditions, it is anticipated that MLC remains in a dephosphorylated state, because of excess MLC phosphatase activity despite the potential activation of MLC kinases through Ca2+/CaM. (B) In thrombin-pre-treated BCECs, the concerted activation of IP3/Ca2+ signaling, leading to the activation MLC kinase combined with the activation RhoA/Rho kinase, leading to the inhibition of MLC phosphatase, leads to an enhanced contractility of the actin/myosin cytoskeleton. The latter seems linked to the Cx43 hemichannels, likely through its C-terminal tail. Increased actin/myosin contractility is proposed to displace the C-terminal tail from the cytoplasmic loop, thereby annihilating loop/tail interactions essential for Cx43-hemichannel opening. As such, Ca2+ signaling triggered by mechanical stimulation will not be able to lead to opening of these “locked”/'closed” Cx43 hemichannels, preventing extracellular ATP release and suppressing ATP-driven Ca2+-wave propagation to neighboring cells. In addition to the effects of the actin/myosin contractility on Cx43 hemichannels, actin/myosin contractility may alter tight junctions and the ability of “head-to-head” docked hemichannels to form gap junction channels.