Abstract
Background
A limitation of mandibular Distraction Osteogenesis (DO) is the length of time required for consolidation. This drawback subjects patients to possible pin-site infections, as well as a prolonged return to activities of normal daily living. Developing innovative techniques to abridge consolidation periods could be immensely effective in preventing these problematic morbidities. Deferoxamine (DFO) is an angiogenic activator that triggers the HIF-1α pathway through localized iron depletion. We previously established the effectiveness of DFO in enhancing regenerate vascularity at a full consolidation period (28 days) in a murine mandibular DO model. To investigate whether this augmentation in vascularity would function to accelerate consolidation, we progressively shortened consolidation periods prior to μCT imaging and biomechanical testing (BMT).
Materials and Methods
Three time points (14d, 21d and 28d) were selected and six groups of Sprague-Dawley rats (n=60) were equally divided into control (C) and experimental (E) groups for each time period. Each group underwent external fixator placement, mandibular osteotomy, and a 5.1mm distraction. During distraction, the experimental groups were treated with DFO injections into the regenerate gap. After consolidation, mandibles were imaged and tension tested to failure. ANOVA was conducted between groups, and p < 0.05 was considered statistically significant.
Results
At 14 days of consolidation the experimental group demonstrated significant increases in Bone Volume Fraction (BVF), Bone Mineral Density (BMD) and Ultimate Load (UL) in comparison to non-treated controls. The benefit of treatment was further substantiated by a striking 100% increase in the number of bony unions at this early time-period (C:4/10 vs. E:8/10). Furthermore, metrics of BVF, BMD, Yield and UL at 14 days with treatment demonstrated comparable metrics to those of the fully consolidated 28d control group.
Conclusion
Based on these findings, we contend that augmentation of vascular density through localized DFO injection delivers an efficient means for accelerating bone regeneration without significantly impacting bone quality or strength.
Keywords: Mandibular Distraction Osteogenesis, Consolidation, Angiogenesis, Deferoxamine
(1.1) Background
Mandibular distraction osteogenesis (DO) has emerged as an efficacious surgical technique for the treatment of congenital retrognathia, micrognathia and mandibular hypoplasia. Increasing surgeon experience and device innovation has allowed for the expansion of this attractive technique throughout the craniofacial skeleton to allow for the correction of maxillary hypoplasia, midface hypoplasia and craniosynostosis [1-4]. Despite these clinical advancements, the length of time required for bone consolidation continues to be a significant limitation to the widespread use of this procedure [5].
In contrast to fracture healing in which bone takes six to eight weeks to heal, DO requires device manipulation during lengthening and a subsequent consolidation period that is proportional to the amount of bone lengthening. This consolidation period ranges from approximately two to six months depending on the desired length and anatomic location of distraction [3,6].
Numerous factors play a role in the success of DO, with blood flow as paramount to the formation of a healthy union. The importance of sufficient blood flow to the fractured ends during consolidation is related to osteoblast survival [7-9]. Osteoblast survival, in turn, relies on the close proximity of these cells to nutrient vessels (<100μm) [10,11]. Studies of non-unions demonstrate ischemic, fibrous tissue; whereas healthy unions often demonstrate high levels of neovascularization and active osteogenesis [12]. The dense vascular network in the craniofacial skeleton allows for a high success rate of DO; however, improved angiogenesis would reasonably lead to faster consolidation. Improving vascularity would not only enhance osteogenesis through improved blood flow to osteoblasts, but vascular tissues themselves also secrete osteogenic cytokines such as BMP-2 and vasculogenic cytokines such as VEGFA, which have also been shown to enhance osteogenesis [13].
Other novel treatment methods of distraction optimization have been reported; however, considerable regulatory hurdles have prevented translation of these methods to the clinical arena [14-17]. A therapeutic paradigm focused on the optimization of vascularity with an established, FDA approved therapy may be a strategy for distraction optimization with real translational potential.
Recent tissue engineering strategies have shed light on the use of angiogenesis and vascular augmentation as mechanisms to enhance bone regeneration in long bone murine models. Investigators have utilized deferoxamine, an iron chelator with angiogenic properties, to trigger angiogenesis and report quantifiable enhancements in vascularity at 14 days and bone quality at 28 days–a widely accepted normal time-point for expected full consolidation [9,18]. Deferoxamine exerts its angiogenic function through the stimulation of the HIF-1 α pathway. Iron is a co-factor in a reaction that leads to the degradation of HIF-1 α. In the absence of iron, the production of HIF-1 α becomes constitutive resulting in an accumulation of the factor in the nucleus. HIF-1 α then dimerizes and begins a transcriptional cascade of events leading to the production of VEGF, as well as other downstream angiogenic factors. Deferoxamine simply removes iron from this equation, thereby triggering angiogenesis and subsequently, new blood vessel formation [18-20].
We previously reported on the ability of deferoxamine to successfully augment vascularity and bone cellularity even beyond that of the normal regenerative response in our rat model of mandibular DO at a full 28d consolidation period. Our results demonstrated a significant 40% increase in vessel number and a 45% increase in osteocytes within the regenerate site [21,22].
In order to expand on our findings, we aim to investigate the therapeutic potential of deferoxamine to substantially shorten consolidation periods. Here we examine its utility in our model at three consecutive time-points (14d, 21d and 28d). We posit that the addition of deferoxamine will allow for successful consolidation at substantially shortened time-points (14 and 21 days) without compromising regenerate quality or mechanical strength when compared to a normal, fully consolidated regenerate (28 days).
(1.2) Materials and Methods
(1.2.1) Experimental Design
Twelve-week-old male Sprague Dawley rats weighing approximately 400 g were paired in cages and maintained in a pathogen-free environment on a 12-hour light/dark schedule. Six groups of animals (n=60) were equally divided into control (C) and experimental (E) groups for each time-period (n=10 per group). Three time-points of consolidation were chosen (14d, 21d and 28d). The 28d time-point was selected as a full, standard consolidation period based on what is widely accepted in the literature and our previous experimentation with this animal model [9,18,21,23,24]. During a 7-day acclimation period before surgery, rats were fed standard hard chow and water ad libitum. Animals underwent mandibular osteotomy and placement of an external distraction device. Experimental groups received deferoxamine injections during the distraction period. Subsequently, all groups were subjected to a variable consolidation period prior to outcome analysis (Figure 1 top). All animal experimentation was conducted in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4 and approved by the University of Michigan Animal Care and Use Committee.
Figure 1.
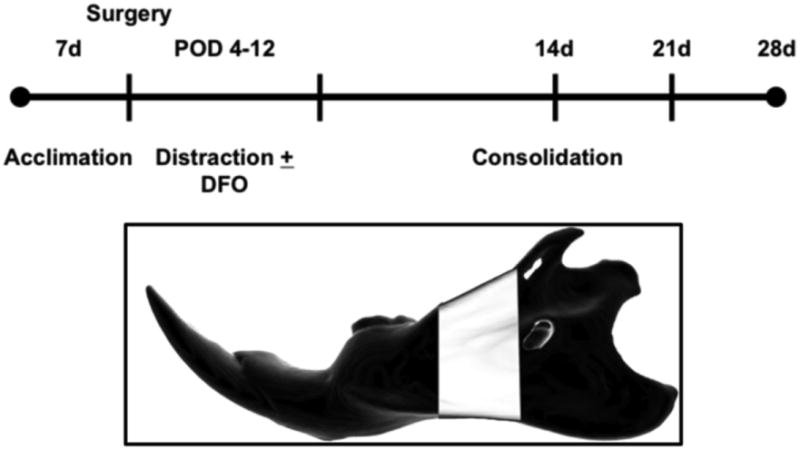
(Top): Experimental timeline illustrating seven days of acclimation, osteotomy, distraction over an eight day period, and three variable consolidation periods (14, 21 and 28d). Note that deferoxamine is given to animals in the experimental group after surgery on POD 4-12. (Bottom): Schematic left hemi-mandible demonstrating the region of interest highlighted in white.
(1.2.2) Mandibular Osteotomy
The techniques for mandibular osteotomy and DO were previously described. Briefly, pre-operative subcutaneous injections of gentamycin (5 mg/kg), buprenorphine (0.03 mg/kg) and lactated Ringer's solution (25 cc/kg) were administered. Animals were then anesthetized with an inhalational oxygen/isoflurane mixture. The animals were prepared for surgery and an osteotomy was created behind the third molar of the left hemi-mandible. This was subsequently secured with a custom-made titanium external distractor device as previously described [23]. Post-operatively, animals were housed one per cage and fed moist chow with Hill's high-calorie diet (Columbus Serum, Columbus, Ohio). Incisors were clipped when needed due to unopposed lengthening. Incisor growth is accelerated due to the soft diet and induced cross-bite from the unilateral distraction. Animals underwent a group-specific consolidation period (14d, 1d or 28d) before euthanasia and outcome analysis.
(1.2.3) Mandibular Distraction
Animals underwent active distraction from the evening of postoperative day 4 through the evening of postoperative day 12. One 180-degree clockwise turn of the distraction screw resulted in a 0.3 mm separation of the osteotomy fronts. Turning of the screw was done every 12 hours for a total of 17 half turns, which produced a cumulative final gap distance of 5.1mm. No analgesic or sedation was required during distraction. The selection of our gap distance was based on findings from our previous experimentation with this model over the years. A 5.1 mm acute gap in the rat mandible consistently produces non-union (critical size defect), whereas gradual separation to this gap distance reliably heals by a predominantly intramembranous mechanism characteristic of DO [25].
(1.2.4) Deferoxamine Injection
For the experimental groups, deferoxamine (200 μM in 300 μL NS) was locally injected directly into the distraction gap every other day during the active distraction period starting on post-operative day 4 and continuing through post-operative day 12. This dose was derived from an extensive literature search regarding the use of deferoxamine in long bone animal models, as well as our experimentation with this drug in recent years [18-21,26,27]. We implemented the injection of deferoxamine therapy during the active distraction period to coincide with the known augmentation of angiogenesis naturally occurring in response to the mechanical stretching of the callus [7,8,10]. Animals were briefly anesthetized (using an isoflurane bell jar technique) during injection to avoid pain and prevention of motion. The injection was administered directly into the clinically palpable regenerate gap. One hundred fifty micro-liters were administered inferiorly and then superiorly to fill the regenerate gap volume. Methylene blue dye was injected in a subset of animals to ensure drug delivery into the regenerate site as previously described [21].
(1.2.5) Bony Union Analysis
En-bloc dissection of mandibles allowed for assessment of bony union. This was clinically defined as absence of movement across the regenerate site on manual manipulation of the regenerate gap after removal of the fixator device. Union status was subsequently verified with Micro-Computed Tomography (μCT) imaging [28].
(1.2.6) μCT
Images were obtained using 80 kVp, 80 mA and 1100 ms exposures. Three hundred ninety-two projections were taken at a 45-micron voxel size for bone analysis. General Electric's Microview 2.2 software was utilized to define our region of interest (ROI) volume and derive radiomorphometrics. The ROI spans a distance of 5.1mm posterior to the third molar corresponding to the surgical site of osteotomy (Figure 1 bottom). Utilizing the splining tool, only bone within the ROI was selected, ensuring exclusion of surrounding spaces and the incisors. Metrics for bone volume fraction (BVF), bone mineral density (BMD) and tissue mineral density (TMD) were obtained.
(1.2.7) Biomechanical Tension Testing
Mandibles were potted after imaging and loaded to failure in uniaxial monotonic tension at 0.5 mm/s using a servohydraulic 858 Minibionix II testing machine (MTS Systems Corporation; Eden Prairie, MN). Crosshead displacement was recorded by using an external linear variable differential transducer (LVDT; Lucas Schavitts, Hampton, VA), and load data were collected with a 100-lb load cell (Sensotec, Columbus, OH). Data were sampled at 200 Hz on a TestStar system (TestStar IIs System version 2.4; MTS Systems Corporation). Load displacement curves were analyzed for yield load (Y), stiffness (S) and ultimate load (UL), using custom computational code (MATLAB 7.11; Mathworks Inc., Natick, MA).
(1.2.8) Statistical Analysis
Utilizing SPSS v. 20 software, an analysis of variance (ANOVA) with post-hoc Tukey's test was used to determine significance between group means with p < 0.05 considered statistically significant.
(1.3) Results
(1.3.1) μCT Results
Radiomorphometrics collected through μCT imaging revealed the clear effects of therapy at each time point (Figures 2 and 3a-c; Tables 1 and 2).
Figure 2.
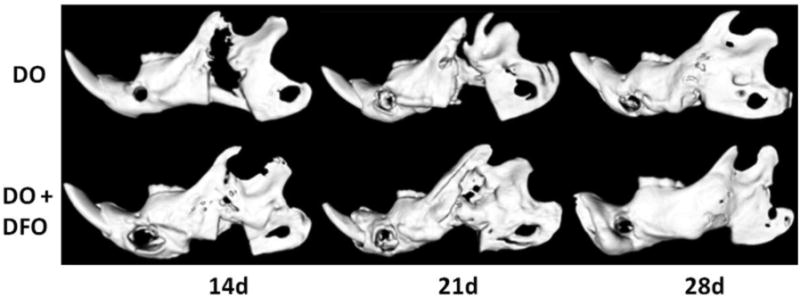
Select μCT images demonstrating the DO group (top row) and the DO + DFO group (bottom row) at each time point. At the earlier 14 and 21d time-points, note the visible augmentation in regenerate radiodensity in the treatment group indicative of accelerated consolidation with therapy.
Figure 3a-c.
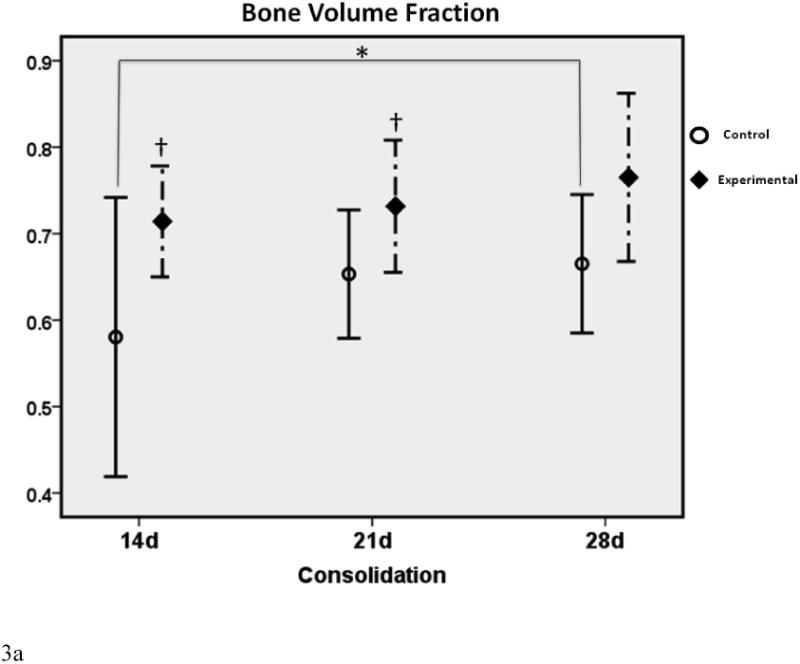
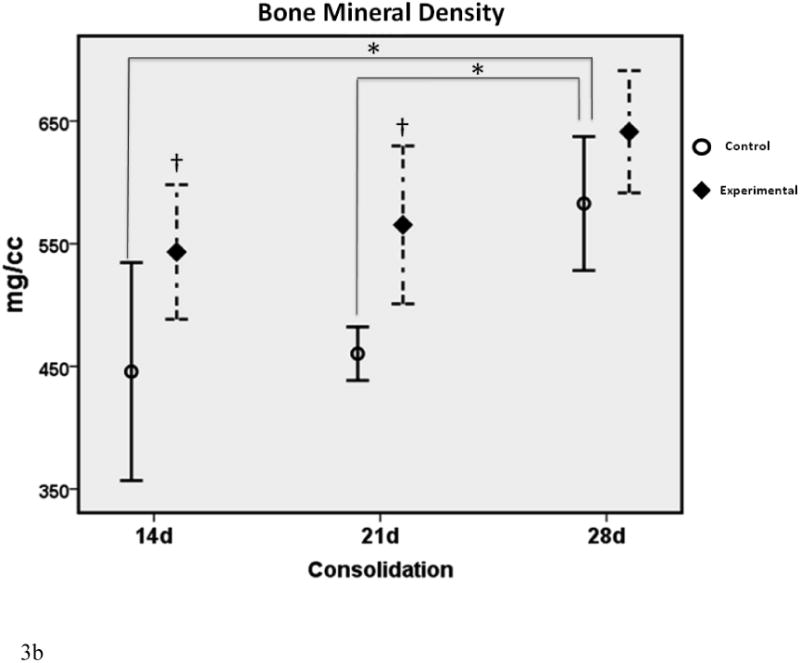
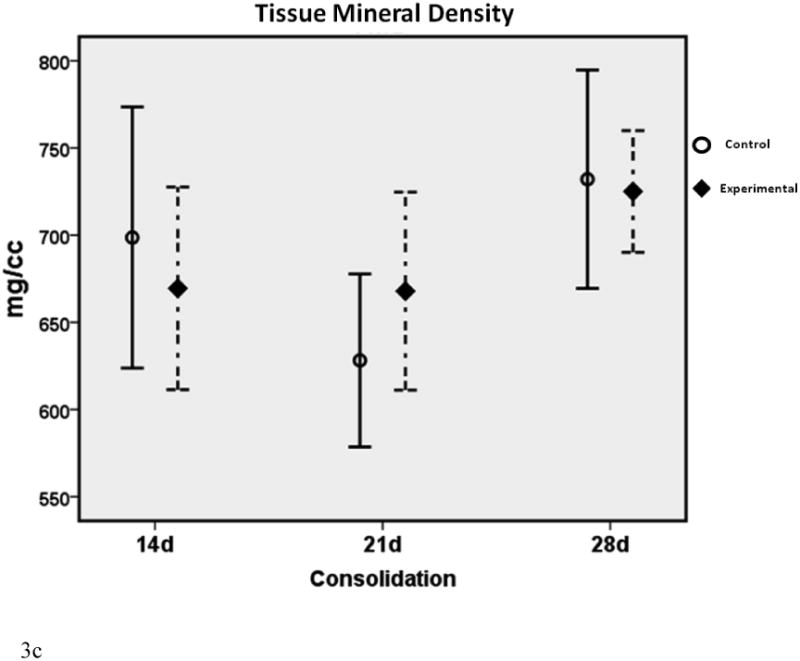
μCT graphs depicting the means ± standard deviations for each group. * denotes statistical significance in comparison to 28d C (p < 0.05). † denotes statistical significance between E and C groups within a single time-point (p < 0.05).
Table 1.
μCT radiomorphometric values as a percentage of the standard full consolidation 28d control values.
| Radiomorphometric Comparison to 28d C | ||||
|---|---|---|---|---|
| %Of 28dC | 14d C vs. 28d C | 14d E vs. 28d C | 21d C vs. 28d C | 21d E vs. 28d C |
| BVF | 77% | 101% | 89% | 109% |
| BMD (mg/cc) | 77% | 96% | 81% | 99% |
| TMD (mg/cc) | 95% | 92% | 87% | 93% |
Table 2.
μCT radiomorphometric values as a percent change from control.
| Radiomorphometric Comparison between Groups within a Time-point | |||
|---|---|---|---|
| %diff | 14d C vs. 14d E | 21d C vs. 21d E | 28d C vs. 28d E |
| BVF | +17% | +10% | +11% |
| BMD (mg/cc) | +25% | +22% | +10% |
| TMD (mg/cc) | -3% | +7% | -1% |
In comparison to the standard 28d fully consolidated control (28d C), radiomorphometric analyses of the non-treated group at 14 days (14d C) demonstrated an expected significant disparity in BVF and BMD (both were 77% of 28d C). This difference was not apparent with deferoxamine therapy, indicating a benefit of treatment at the earliest time-point (14d E BVF = 101% of 28d C; 14d E BMD = 96% of 28d C). At 21 days, a similar effect was observed for BMD, indicating a continued beneficial effect of deferoxamine therapy (21d C = 89% of 28dC vs. 21d E = 109% of 28d C). BVF, however, was no longer significantly decreased between 21d C and 28d C, reflecting the normal effect of increases in regenerate volume and mineralization over time (21d C = 89% of 28d C). Further, when comparing E to C groups within each respective time-point, a significant increase in BMD and BVF was observed at 14 and 21 days. Lastly, throughout the study TMD showed no differences between any of the groups, indicating that neither time nor therapy affected the mineralization of new bone.
In summary:
The effect of therapy was most evident on increases in BVF and BMD at the earliest (14d) time-point, and in BMD at the second (21d) time-point. With therapy, these increases were normalized to fully consolidated levels (28d C).
(1.3.2) Biomechanical Testing Results
Tension testing the mandibles to failure further indicated the effects of therapy at each time point (Figure 4a-c; Tables 3 and 4).
Figure 4.
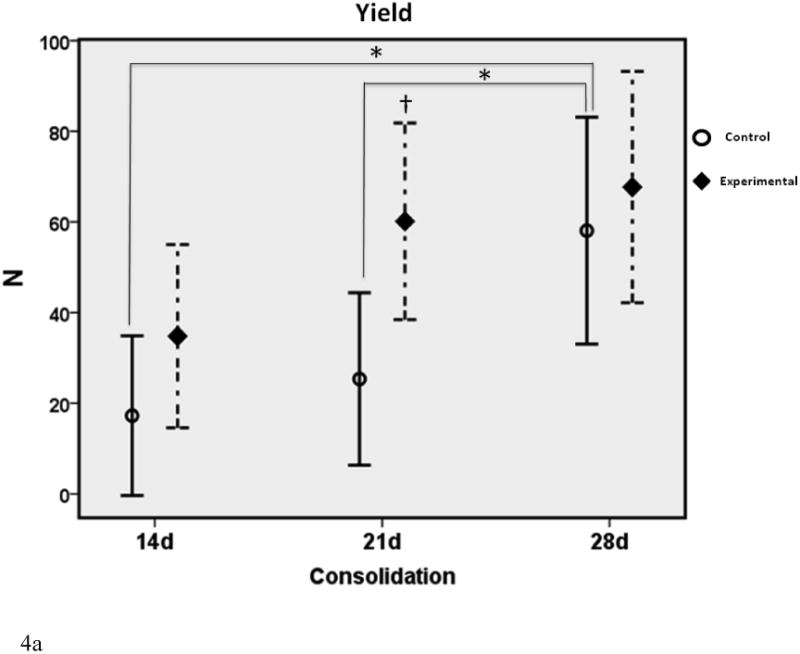
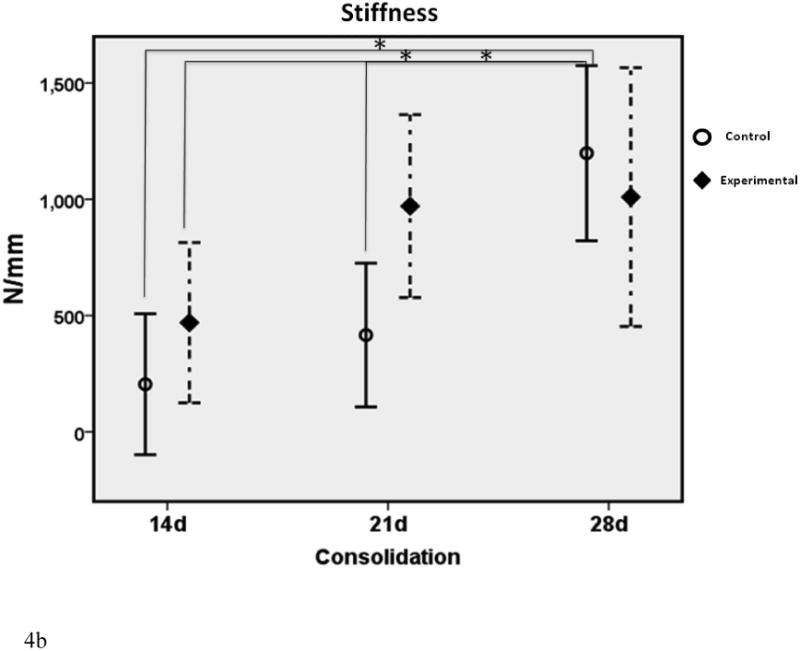
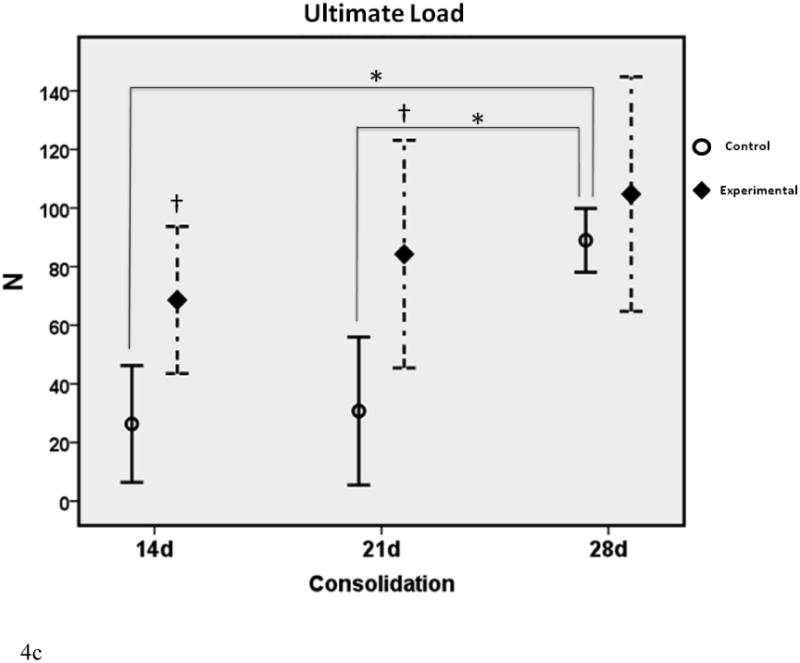
a-c. Biomechanical graphs depicting the means ± standard deviations for each group. * denotes statistical significance in comparison to 28d C (p < 0.05). † denotes statistical significance between E and C groups within a single time-point (p < 0.05).
Table 3.
Biomechanical testing metric values as a percentage of the standard full consolidation 28d control values.
| Biomechanical Metric Comparison to 28d C | ||||
|---|---|---|---|---|
| 14d C vs. 28d C | 14d E vs. 28d C | 21d C vs. 28d C | 21d E vs. 28d C | |
| Y (N) | 28% | 61% | 41% | 103% |
| S (N/mm) | 17% | 39% | 35% | 80% |
| UL (N) | 29% | 76% | 35% | 94% |
Table 4.
Biomechanical testing metric values as a percent change from control.
| Biomechanical Metric Comparison between Groups within a Time-point | |||
|---|---|---|---|
| 14d C vs. 14d E | 21d C vs. 21d E | 28d C vs. 28d E | |
| Y (N) | +118% | +152% | +16% |
| S (N/mm) | +129% | +129% | -16% |
| UL (N) | +165% | +171% | +18% |
In comparison to the standard 28d fully consolidated control, biomechanical analyses demonstrated significantly lower values for Y, S and UL in the non-treated group at 14 days (28%, 17% and 29% of 28d C respectively). This difference was not apparent for Y and UL with therapy in the 14d E group (61% and 76% of 28d C respectively). Despite treatment, S in the 14d E group exhibited a significant disparity from control levels at 28d (39% of 28d C). Twenty-one day control Y, S and UL were still significantly less than 28d C levels, as expected (41%, 35% and 35% of 28d C respectively). These differences were no longer apparent with the addition of therapy to the 21d groups (103%, 80% and 94% of 28dC respectively). When comparing C to E groups within each time-point, treatment groups exhibited increased UL at 14 days and both UL and Yield at 21 days. There were no significant differences between groups within each time-point for S, nor were there any differences observed for Y, S or UL at 28d between C and E groups.
When examining group means over time, it was apparent that control Y, S and UL continued to increase throughout the study. In contrast, the experimental group Y and S reached fully consolidated levels by 21 days and largely plateaued thereafter. Both groups continued to increase in UL over time, although the relative rise was much higher in the control group, particularly between 21 and 28 days. With the exception of S at 28d, experimental means were higher than control means for each metric at each time-point in the study.
In summary:
The effect of therapy was most evident on increases in UL at 14 and 21 days. These values were normalized to fully consolidated levels (28d C) in the experimental group, whereas the non-treated group exhibited a significant disparity.
(1.3.3) Bony Union Results
At the earliest time-point (14d), only 4/10 control mandibles exhibited bony union compared to 8/10 experimental mandibles (Figure 5). Further, unions in the 14d control group exhibited unions due to sparse bony bridging in the regenerate gap when compared to experimental mandibles (Figure 6).
Figure 5.
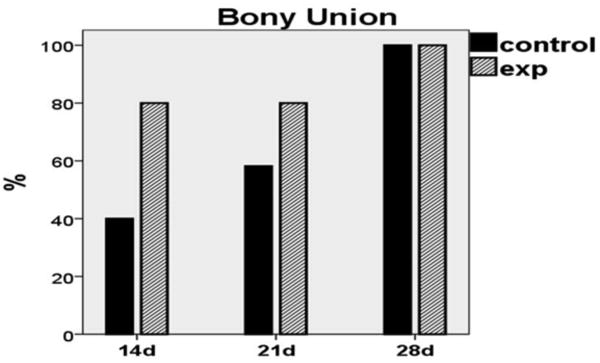
Graph demonstrating bony union rates within groups. Note the doubling of bony unions in the experimental group at 14d when compared to non-treated controls. At 21d, control union rate increased by 20%; however, experimental union rate remained increased by comparison. By 28 days, both groups exhibited 100% bony union rate.
Figure 6.
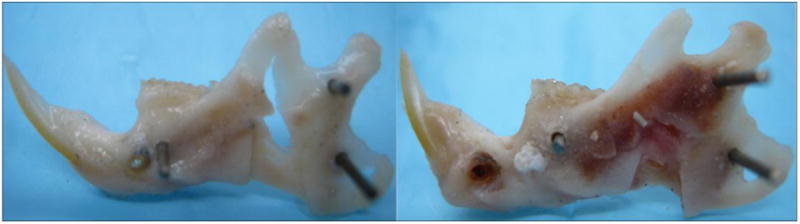
Control (L) and Experimental (R) hemimandibles after 14 days of consolidation. Visible gross enhancements in regenerate quality are apparent at this early time-period.
(1.4) Discussion
(1.4.1)
In the present study we investigated the therapeutic potential of deferoxamine to accelerate regenerate consolidation after mandibular DO. While other methods to accelerate consolidation have been attempted, we specifically aimed to optimize an ongoing and well-established phenomenon occurring during distraction. Angiogenesis and vascular augmentation in response to the mechanical stretching of a callus are critical mechanisms integral to the successful regeneration of bone [10,29-32]. Here we sought to enhance the natural vascular augmentation achieved in distraction by utilizing an angiogenic therapy during the distraction period. Ultimately, we hypothesized that the addition of deferoxamine would enable successful consolidation at substantially shortened time-points without compromising regenerate quality or mechanical strength. In this experiment, quantitative metrics consistently demonstrated the beneficial effects of therapy at abridged time-points.
(1.4.2) Radiomorphometric Data
By 21 days, control BVF had reached a plateau consistent with fully consolidated levels; however, a disparity still existed in the control BMD. It was apparent that therapy continued to impact positive changes in BMD at this time-point, allowing for a beneficial increase in BMD to fully consolidated levels. By 28d of consolidation, statistical differences between groups no longer existed. We posit that the untreated bone finally “caught up” to the accelerated process resulting from DFO treatment.
In summary:
Deferoxamine therapy impacted an acceleration of mineral deposition leading to increased mineral volume within the regenerate at the earlier time-periods when compared to control.
(1.4.3) Biomechanical Data
The greatest effect of therapy was observed at the second time-period where all metrics (Y, S and UL) reached fully consolidated levels in the experimental groups. This was not observed in the control groups. The time between the second and third time-points (day 21-28) also appeared to be the most critical period for normal regenerate development from a biomechanical perspective. While control mandibles only marginally increased in Y, S and UL between the first and second time-periods (days 14-21; Δ = +9N, + 211N/mm and + 5N respectively), the biggest gains were observed between the second and third time-periods (Δ = +36N, + 782N/mm and + 58N respectively). This pattern was not observed in the experimental group. Y and S increased notably between the first and second time period, but achieved control levels and plateaued thereafter. Experimental UL steadily increased throughout the study, presumably highlighting the effect of continued regenerate organization as a dominant factor, as opposed to increases in UL due to mineral deposition and callus enlargement over time as observed in the control group. These results reflect that the biomechanical integrity of the treated regenerate is largely established by the 21d time-point, whereas substantial increases in regenerate mineralization and associated mechanical integrity are ongoing in the control groups throughout the experiment.
In summary:
Without therapy, the regenerate was still experiencing large gains in biomechanical strength throughout the study, while treated mandibles achieved the bulk of biomechanical integrity by 21 days.
(1.4.4) Bony Union Assessment
On gross inspection of the dissected mandibles, we observed a natural progression of bony union formation in the control group over time. At each time-point the number of unions increased, ultimately reaching a 100% bony union rate by 28 days (4/10, 6/10, 10/10 respectively). Conversely, the experimental group achieved a higher union rate than the control group at both earlier time periods (8/10 at 14 and 21 days). By 28 days, both groups exhibited 100% union rate (10/10). Taken together, these findings highlight the effect of therapy in the early formation of a clinically relevant benchmark–the formation of bony union.
In summary:
Treated mandibles exhibited twice as many unions at the earliest time period when compared to untreated controls.
(1.4.5)
All of our findings amalgamate to our conclusion that deferoxamine functions to expedite consolidation and healing during mandibular DO.
Despite our promising findings, several clinically relevant limitations should be addressed. While we saw increases in bony union at the earlier time-periods, it is important to note that 100% union rate was not achieved with therapy at 14 and 21 days. Further, when regarding biomechanical data, statistical significance does not directly translate to clinical relevance. For example, while our 14d experimental ultimate load was not statistically different than fully consolidated controls, there was in fact a disparity of 63N. This difference, while not statistically significant, may have considerable clinical implications. Due to these limitations, future directions may consider optimization of early consolidation with the addition of synergistic therapies. Finally, while our results are promising with regards to the acceleration of consolidation, it is important to note the potential to over-accelerate distraction and cause premature consolidation. With regards to this potential complication, future investigations may consider the controllability of deferoxamine by examining dose-response relationships between the amount of injected deferoxamine and the rate of consolidation in this model.
(1.4.6) Conclusions
Our results substantiate the assertion that angiogenic and vascular enhancements during DO serve to stimulate powerful mechanisms that can function to hasten bone regeneration and shorten lengthy consolidation periods. This study demonstrates quantifiable increases in radiomorphometric and biomechanical parameters of regenerate healing at significantly abridged time-points during consolidation due to vascular augmentation with deferoxamine therapy. While further studies are needed to address the aforementioned limitations of this work, based on our findings, we contend that this therapeutic strategy should be considered as a translational option for the acceleration of bone healing in patients undergoing DO.
Highlights.
We demonstrate the degree of acceleration of consolidation during distraction osteogenesis with the utility of angiogenic deferoxamine therapy. > We report quantifiably enhanced radiomorphometrics of bone quality and metrics of biomechanical strength at abridged time-points that are comparable to fully consolidated controls. > Our findings suggest that augmentation of vascular density through localized DFO injection delivers an efficient means for accelerating bone regeneration without significantly impacting bone quality or strength.
Acknowledgments
Sponsored by the American Society of Maxillofacial Surgeons® grant award for proposal titled: “The Effect of Deferoxamine on the Rate of Consolidation in Distraction Osteogenesis” to Alexis Donneys. Further funding was provided by NIH RO1 CA 12587–01 to S. R. Buchman and NIH T32-GM 008616, for Alexis Donneys. The authors would like to thank Charles Roehm for fabrication of fixator devices.
Financial Support: Funding Support provided by: American Society of Maxillofacial Surgeons Grant Award to A Donneys for proposal titled “The Effect of Deferoxamine on the Rate of Consolidation in Distraction Osteogenesis”.
NIH T32-GM 008616, for A. Donneys
NIH RO1 CA 12587-01 to S. R. Buchman.
Footnotes
Disclosure: All authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polley JW, Figueroa AA. Management of severe maxillary deficiency in childhood and adolescence through distraction osteogenesis with an external, adjustable, rigid distraction device. J Craniofac Surg. 1997;8:181–6. doi: 10.1097/00001665-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Mofid MM, Manson PN, Robertson BC, Tufaro AP, Elias JJ, Vander Kolk CA. Distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg. 2001;108:1103–14. doi: 10.1097/00006534-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Toth BA, Kim JW, Chin M, Cedars M. Distraction osteogenesis and its application to the midface and bony orbit in craniosynostosis syndromes. J Craniofac Surg. 1998;9:100–13. doi: 10.1097/00001665-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wiltfang J, Hirschfelder U, Neukam FW, Kessler P. Long-term results of distraction osteogenesis of the maxilla and midface. Br J Oral Maxillofac Surg. 2002;40:473–9. doi: 10.1016/s0266435602002474. [DOI] [PubMed] [Google Scholar]
- 5.Yonehara Y, Hirabayashi S, Sugawara Y, Sakurai A, Harii K. Complications associated with gradual cranial vault distraction osteogenesis for the treatment of craniofacial synostosis. J Craniofac Surg. 2003;14:526–8. doi: 10.1097/00001665-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Andersen K, Nørholt SE, Küseler A, Jensen J, Pedersen TK. A retrospective study of cleft lip and palate patients satisfaction after maxillary distraction or traditional advancement of the maxilla. J Oral Maxillofac Surg. 2012;3:e3. doi: 10.5037/jomr.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai-Aql ZS, Alagl S, Graves DT, Gerstenfeld LC, Einhorn T. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–18. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glowacki J. Angiogenesis in fracture repair. Clin Orthop Relat Res. 1998;355(Suppl):S82–S89. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 9.Donneys A, Tchanque-Fossuo CF, Farberg AF, Deshpande SD, Buchman SR. Bone regeneration in distraction osteogenesis demonstrates significantly increased vascularity in comparison to fracture repair in the mandible. J Craniofac Surg. 2012;23:328–32. doi: 10.1097/SCS.0b013e318241db26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994;301:124–31. [PubMed] [Google Scholar]
- 11.Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang SY. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30:508–17. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20:1114–24. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara H, Hogan DE, Morgan EF, Mortolock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–80. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai JP, Wang FS, Hung CM, Wang CJ, Huang CJ, Kuo YR. Extracorporeal shock wave accelerates consolidation in distraction osteogenesis of the rat mandible. J Trauma. 2010;69:1252–8. doi: 10.1097/TA.0b013e3181cbc7ac. [DOI] [PubMed] [Google Scholar]
- 15.Cillo JE, Jr, Gassner R, Koepsel RR, Buckley MJ. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: Implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:147–54. doi: 10.1067/moe.2000.107531. [DOI] [PubMed] [Google Scholar]
- 16.Ashinoff RL, Cetrulo CL, Jr, Galiano RD, Dobryansky M, Bhatt KA, Ceradini DJ, Michaels J, McCarthy JG, Gurtner GC. Bone morphogenic protein-2 gene therapy for mandibular distraction osteogenesis. Ann Plast Surg. 2004;52:585–90. doi: 10.1097/01.sap.0000123023.28874.1e. [DOI] [PubMed] [Google Scholar]
- 17.Kontaxis A, Abu-Serriah M, Ayoub AF, Barbenel JC. Mechanical testing of recombinant human bone morphogenetic protein-7 regenerated bone in sheep mandibles. Proc Inst Mech Eng H. 2004;218:381–8. doi: 10.1243/0954411042632135. [DOI] [PubMed] [Google Scholar]
- 18.Shen X, Wan C, Ramaswamy G, Mavalli M, Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhart A, Clemens TL, Gilbert SR. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxia-inducible factor-1HIF 1-α pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–91. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–61. [Google Scholar]
- 21.Donneys A, Farberg AS, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Deferoxamine enhances the vascular response of bone regeneration in mandibular distraction osteogenesis. Plast Reconstr Surg. 2012;129:850–6. doi: 10.1097/PRS.0b013e31824422f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farberg AS, Razdolsky ER, Jing XL, Zehtabzadeh AJ, Tchanque-Fossuo CN, Donneys A, Deshpande SS, Monson LA, Buchman SR. Deferoxamine Treatment Increases Osteocyte Count in Mandibular Distraction Osteogenesis. Plast Reconstr Surg. 2010;126:8. [Google Scholar]
- 23.Buchman SR, Ignelzi MA, Jr, Radu C, Wilensky J, Rosenthal AH, Tong L, Rhee ST, Goldstein SA. A unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–9. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Farberg AS, Sarhaddi D, Razdolsky ER, Donneys AD, Deshpande SS, Buchman SR. Deferoxamine Enhances Bone Regeneration in Irradiated Mandibular Distraction Osteogenesis. Plast Reconstr Surg. 2012;130:73. doi: 10.1097/01.prs.0000438050.36881.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: Evidence for mechanotransduction. Plast Reconstr Surg. 2003;111:211–22. doi: 10.1097/01.PRS.0000033180.01581.9A. [DOI] [PubMed] [Google Scholar]
- 26.Farberg AS, Jing XL, Monson LA, Donneys A, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone. 2012;50:1184–7. doi: 10.1016/j.bone.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donneys A, Weiss DM, Deshpande SS, Ahsan S, Tchanque-Fossuo CN, Sarhaddi D, Levi B, Goldstein SA, Buchman SR. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52:318–25. doi: 10.1016/j.bone.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 29.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part I. The influence of stability of fixation and soft tissue preservation. Clin Orthop Relat Res. 1989;238:249–81. [PubMed] [Google Scholar]
- 30.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–85. [PubMed] [Google Scholar]
- 31.Ilizarov GA, Ledyaev VI. The replacement of long tubular bone defects by lengthening distraction osteotomy of one of the fragments. Clin Orthop Relat Res. 1992;280:7–10. [PubMed] [Google Scholar]
- 32.Aronson J. Experimental and clinical experience with distraction osteogenesis. Cleft Palate Craniofac J. 1994;31:473–81. doi: 10.1597/1545-1569_1994_031_0473_eacewd_2.3.co_2. [DOI] [PubMed] [Google Scholar]


