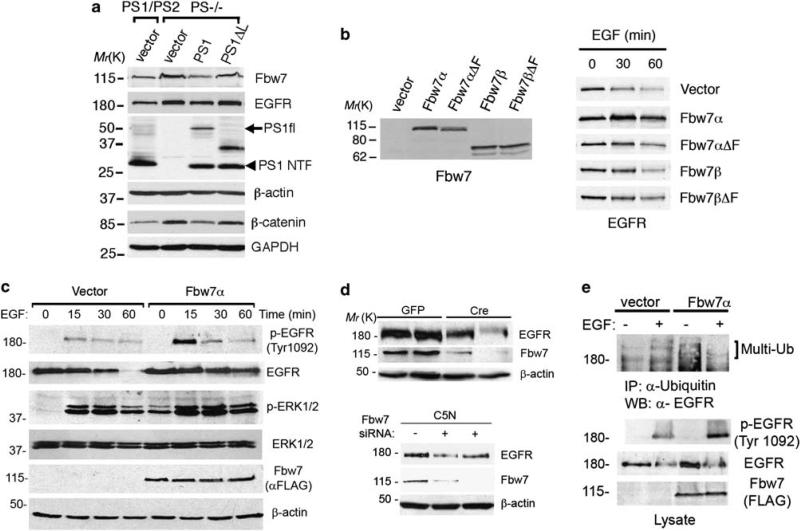
Figure 5.
The ubiquitin ligase Fbw7 stabilizes EGFR expression and signaling. (a) Stable pools of PS1/PS2 or PS–/– fibroblasts expressing human PS1, PS1Δloop or empty vector were selected and generated as described in Materials and methods section. Expression of PS1 but not PS1Δloop in PS–/– fibroblasts reduces the levels of Fbw7 and EGFR. Abnormal levels of β-catenin in the cytosolic fraction of PS–/– fibroblasts were decreased after PS1 expression (bottom blots). (b) 293T cells were transiently transfected with vector, Fbw7α, Fbw7β or the loss of function mutants lacking the F-box domain Fbw7αΔF and Fbw7βΔF for 24 h. Expression of Fbw7 in non-treated cells (left panel; Ab12292 antibody) or EGFR (right panel) in cells treated with EGF (150 ng/ml) for 0–60 min were detected by western blotting. (c) Levels of EGFR and phosphorylated EGFR (Tyr 1092) or ERK1/2 after treatment with EGF were examined by immunoblotting in 293T cells transiently transfected with vector or Flag-Fbw7α. Fbw7α increases phosphorylation and stability of EGFR and enhances sustained ERK1/2 phosphorylation. (d) Top: Cre recombinase-mediated deletion of Fbw7 reduced EGFR levels in two independent Fbw7 f/f fibroblast lines. Bottom: Epithelial C5N cell lines stably expressing pSuper Fbw7 small interfering RNAs (+) showed reduced expression of EGFR compared with pSuper (–) transfected cells. (e) 293T cells were transiently transfected with vector or Flag-Fbw7α and incubated in the absence (–) or presence (+)of EGF on ice for 15 min. Immunoprecipitation/western blotting revealed increased phosphorylation and ubiquitination of EGFR in cells expressing Fbw7.