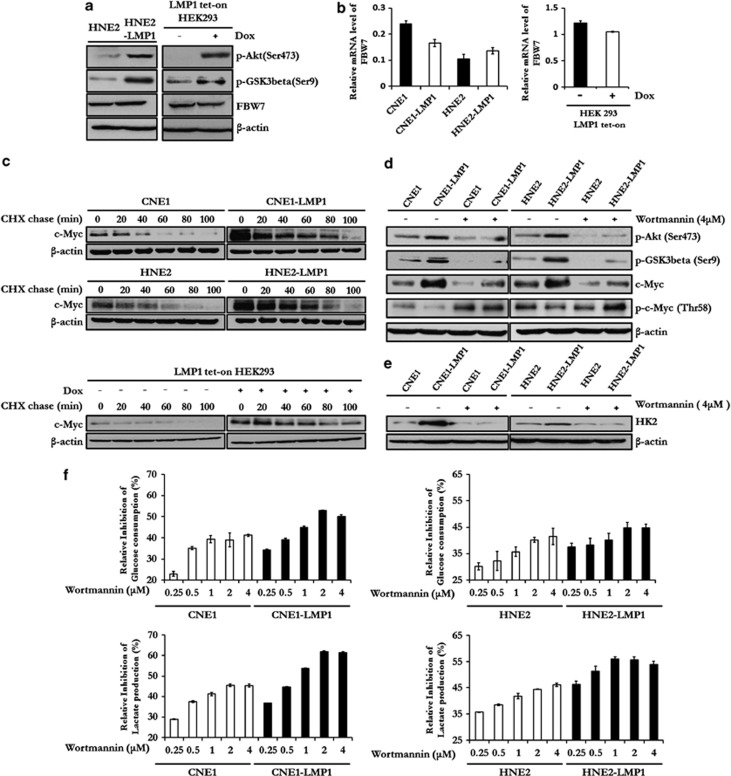
Figure 7.
LMP1-mediated attenuation of the PI3-K/Akt-GSK3beta-FBW7 signaling axis results in stabilization of c-Myc and upregulation of glycolysis. (a) Immunoblotting was used to analyze the expression level of FBW7 and the phosphorylation level of Akt and GSK3beta in HEN2, HNE2-LMP1 and LMP1 tet-on HEK293 cells. β-Actin served as an internal control to verify equal loading of proteins. (b) qRT–PCR was performed to determine the mRNA level of fbw7 in LMP1-overexpressing cells and their parental cells, and LMP1 tet-on HEK293 cells. β-Actin was used as a control to confirm equal loading of cDNAs. Data are shown as means±s.d. of three experiments. (c) The effect of LMP1 on stability of c-Myc in NPC cells and LMP1 tet-on HEK293 cells was examined by the cycloheximide chase assay, cells were treated with cycloheximide (20 μg/ml) for the indicated times. c-Myc expression levels were determined by immunoblot analysis. β-Actin served as an internal control to verify equal loading of proteins. (d) The effect of Wortmannin treatment on c-Myc expression and phosphorylation of Akt (Ser473), GSK3beta (Ser9) and c-Myc (Thr58) was determined by immunoblot analysis. β-Actin served as an internal control to verify equal loading of proteins. (e) The effect of Wortmannin treatment on HK2 expression was determined by immunoblot analysis. β-Actin served as an internal control to verify equal loading of proteins. (f) CNE1, CNE1-LMP1, HNE2 and HNE2-LMP1 cells were treated with Wortmannin at the indicated doses. The relative levels of glucose consumption and lactate production rate were examined in these cell lines using the Automatic Biochemical Analyzer. Data are shown as means±s.d. of three experiments.