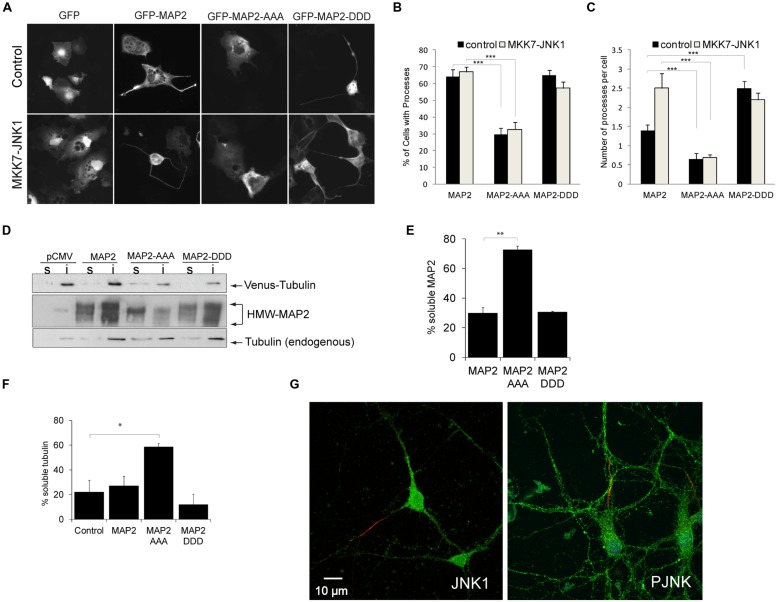
FIGURE 2.
PRD phosphorylation of MAP2 is necessary and sufficient for microtubule stabilization and MAP2-induced protrusion growth. (A) COS-7 cell were transfected with GFP, GFP-MAP2, GFP-MAP2T1619D,T1622D,T1625D (henceforth GFP-MAP2-DDD), or GFP-MAP2T1619A,T1622A,T1625A (henceforth GFP-MAP2-AAA), with or without MKK7-JNK1 as shown. GFP-MAP2-induced protrusion growth while MKK7-JNK1 alone did not. (B) The proportion of cells that generated processes upon 48 h expression of MAP2 variant is shown. GFP-MAP2-AAA expressing cells failed to produce protrusions. (C) The number of processes per cell was counted. Expression of MKK7-JNK1 augmented the number of MAP2-generated processes. GFP-MAP2-DDD alone induced significantly more processes than did GFP-MAP2. (D) Fibroblasts transfected with Venus-tubulin together with GFP, GFP-MAP2, GFP-MAP2T1619A,T1622A,T1625A (MAP2-AAA), or GFP-MAP2T1619D,T1622D,T1625D (MAP2-DDD) were permeablized and the distribution of MAP2 or Venus-tubulin to the TX-100 soluble or insoluble fractions were quantified using immunoblotting. (E) Quantified data shows that GFP-MAP2T1619A,T1622A,T1625A partitions to the soluble phase, while GFP-MAP2T1619D,T1622D,T1625D remains in the TX-100 insoluble phase. (F) Quantitative data on Venus-tubulin stability. GFP-MAP2T1619D,T1622D,T1625D stabilizes microtubules whereas GFP-MAP2T1619A,T1622A,T1625A induces microtubule destabilization. (G) Polarized neurons at 12 days in vitro were immunostained for JNK1 (green) and ankyrin G (red; left panel) to highlight the axon, and P-JNK (green) and ankyrin G (red), right panel. JNK1 and P-JNK immunoreactivity was detected in both axonal and dendritic compartments. For all histograms, means ± SEMs are shown. Significance levels are in each instance compared to control conditions (GFP-MAP2). *p< 0.05; **p< 0.01; ***p< 0.005.