Abstract
Autoimmune enteropathy (AIE) is a rare disorder characterized by severe diarrhea and small intestinal mucosal atrophy resulting from immune-mediated injury. It remains a challenging diagnosis due to its clinicopathologic variability. In order to better understand its histopathological features, we describe the gastrointestinal biopsy findings of 25 patients, including children and adults. The most common finding on small intestinal biopsy (13/25 cases, 52%) was villous blunting, expansion of the lamina propria by mixed but predominantly mononuclear inflammation, and neutrophilic cryptitis with or without crypt microabscesses. In 5 cases (20%), the duodenum exhibited changes indistinguishable from celiac disease, with villous blunting and intraepithelial lymphocytosis. Increased crypt apoptosis with minimal inflammation, resembling acute Graft-versus-Host Disease (GvHD), was observed in 4 cases (16%). The remaining 3 cases (12%) exhibited a mixture of 2 or more of the above patterns. Mucosal abnormalities outside the small intestine were present in all 24 cases with available biopsies (100%), with the stomach most commonly affected (19/22 cases, 86%), followed by the colon (14/22, 64%) and esophagus (5/18, 28%). Findings in non-small intestinal sites were variable and included mixed active and chronic inflammation, chronic inflammation alone, intraepithelial lymphocytosis, and increased apoptosis resembling acute GvHD. In summary, AIE most commonly presents as an active enteritis with villous blunting and expansion of the lamina propria by mixed inflammation. Mucosal abnormalities are frequently seen elsewhere in the gut. AIE may be thus better regarded as a pan-gastrointestinal autoimmune disorder, and biopsies from sites other than the small intestine may greatly facilitate its diagnosis.
Keywords: autoimmune enteropathy, autoimmune enteritis, IPEX syndrome, celiac disease, Graft-versus-Host disease
INTRODUCTION
Autoimmune enteropathy (AIE) is an uncommon disorder characterized by protracted diarrhea, small intestinal villous atrophy, lack of response to dietary exclusion, and evidence of autoimmunity(1-3). The clinical and pathological features of AIE are variable. Although it is primarily a pediatric disease, with an estimated incidence at less than 1 in 100,000 infants(3), it is now well recognized to occur in adults as well(4).
AIE appears to result from dysregulation of gut humoral and immune function. Genetic mutations that result in T-cell overactivity underlie two syndromic forms of AIE: the Immune dysregulation, Polyendocrinopathy, Enteropathy, and X-linked (IPEX) syndrome, and the Autoimmune phenomena, Polyendocrinopathy, Candidiasis, and Ectodermal Dystrophy syndrome (APECED), also known as Autoimmune Polyglandular syndrome (APS-1)(2, 3, 5). IPEX syndrome in particular is caused by loss-of-function mutations in Forkhead box p3 (FOXP3), a transcriptional regulator required for normal development and function of regulatory T-cells (Treg)(6). Loss of FOXP3 activity leads to immune overactivity in response to antigen stimulation and cellular injury via CD4+ effector T cells(5). IPEX syndrome classically presents early in infancy with AIE, insulin-dependent diabetes mellitus, and cutaneous manifestations such as eczema, atopic dermatitis, or alopecia(3, 5).
Aberrant expression of HLA class II molecules by enterocytes has also been documented in AIE(7, 8), and circulating autoantibodies to intestinal epithelial cells (goblet cells or enterocytes) are often detected in AIE patients(1, 4, 9). However, widespread consensus regarding the pathogenic and diagnostic significance of such autoantibodies is lacking. While some have argued for the presence of autoantibodies as a diagnostic criterion(4), other studies have shown that they are not specific for AIE(10, 11). In everyday clinical practice, testing for these antibodies remains difficult and expensive.
Beyond the diagnostic requirement for villous blunting, the small intestinal histopathologic features of AIE are variable. Reported findings include mixed inflammatory infiltrates, prominent crypt epithelial apoptosis similar to acute Graft-vs-Host Disease, and morphological changes largely indistinguishable from celiac disease(4, 7, 9, 12, 13). Goblet cells and Paneth cells may be substantially decreased in numbers or completely absent(4, 7, 9, 13). Although AIE is traditionally considered a disease of the small intestine, other sites in the tubular gut may be affected as well(4, 7, 9, 13, 14). In the absence of truly specific histopathologic findings, misdiagnosis and delayed diagnosis of AIE remain common(2, 3). In order to better understand the spectrum of histopathologic features of AIE in the tubular gut, we describe here the gastrointestinal biopsy findings in 25 patients, which represents the largest series of AIE patients reported to date. The most common pattern of injury seen in the small intestine is an active enteritis with prominent villous blunting, lymphoplasmacytic expansion of the lamina propria, and neutrophilic cryptitis, sometimes with crypt microabscesses.
MATERIALS AND METHODS
25 cases of AIE were identified from 2 institutions: Royal Brisbane and Women’s Hospital, Brisbane, Queensland, Australia and Massachusetts General Hospital, Boston, MA, USA. Cases were identified either prospectively from inpatients seen at the institution, or retrospectively from the pathology files. Patients were diagnosed with AIE based on the diagnostic criteria proposed by Unsworth and Walker-Smith(1): protracted diarrhea, small intestinal villous atrophy, lack of response to dietary therapy, and evidence of autoimmunity in the form of circulating autoantibodies to gut epithelium and/or associated autoimmune diseases. In some patients, clinical response to immunosuppressive therapy was considered evidence of autoimmunity by our clinical colleagues. All available endoscopic biopsies of the gastrointestinal tract were reviewed. Biopsies were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 5 micron thickness, and stained with hematoxylin and eosin. One case was processed at an outside institution and reviewed in consultation at Massachusetts General Hospital. Villous blunting in small intestinal biopsies was graded on the basis of villous-to-crypt ratio as follows: mild, between 2:1 and 3:1; moderate, between 2:1 and 1:1; severe, less than 1:1(15). The diagnostic criterion for increased crypt epithelial apoptosis was >1 apoptotic figure per 10 glands or crypts(16). Where available, immunohistochemical stains for Helicobacter pylori were examined. Immunohistochemical stains for gastrin and chromogranin were performed on gastric biopsies in one case (case 8). Demographic, clinical, and treatment data were obtained from the available medical records.
Duodenal biopsies were classified on basis of the predominant histological pattern as:
Active chronic duodenitis (ACD): villous blunting, expansion of the lamina propria by mixed but predominantly mononuclear inflammation (consisting of lymphocytes, plasma cells, and eosinophils), and neutrophilic cryptitis (with or without crypt abscesses); with or without increased apoptosis in crypt epithelium. Plasma cells were the predominant cell within the lamina propria inflammatory infiltrate.
Celiac disease-like (Celiac-like): villous blunting and marked increase in intraepithelial lymphocytes in surface epithelium (>40 IELs/100 enterocytes)(15).
Graft-versus-Host Disease-like (GvHD-like): increased apoptosis in crypt epithelium (>1 apoptotic figure per 10 crypts)(16), with or without crypt dropout, with minimal inflammation.
Mixed/no predominant pattern: admixture of ≥2 patterns or insufficient features to qualify for any the above 3 patterns.
A duodenal biopsy was not available in one case (case 13). In this instance, ileal biopsy findings were used to classify the small intestinal histological pattern, applying the same criteria described above.
RESULTS
Demographic and clinical features
25 patients were identified, comprising 14 males (56%). The age at the time of diagnosis ranged from 2 months to 80 years. 8 patients were adults (≥18 years; 32%) and 14 were 6 years of age or less (56%). There were 2 patients with IPEX syndrome (9%) and one patient with probable DNA ligase 4 deficiency (4%). All patients presented with intractable or protracted diarrhea. Among other signs and symptoms at presentation, weight loss was common in adults 6 of 8 cases, 75%). Other autoimmune conditions included diabetes mellitus, autoimmune hepatitis, autoimmune haemolytic anemia, rheumatoid arthritis, steroid-responsive uveitis, and thyroid disease. One patient had a family history of AIE.
Anti-enterocyte autoantibodies were detected in 4 of 5 patients tested (80%). Anti-goblet cell antibody testing was not available. Other autoantibodies were detected in 10 of 19 patients tested (53%), and included antinuclear antibody (ANA; the most common, seen in 9 of 16 patients, 56%), antigliadin antibody, anti-cytoplasmic antibody (ANCA), extractable nuclear antibodies (eNA - Ro, La, Sm, RNP, Jo-1, Scl-70, dsDNA), anti-parietal cell antibody, anti-mitochondrial antibody (AMA), anti-liver kidney microsomal (anti-LKM) antibody, anti-smooth muscle antibody (SMA), rheumatoid factor (RF), anti-pancreatic islet cell antibodies, anti-cyclic citrullinated peptide (anti-CCP) antibody, and anti-glutamic acid decarboxylase (anti-GAD) antibody.
Histopathologic findings in the duodenum (Table 1)
Table 1.
Histopathologicalfeatures of AIE on gastrointestinal biopsy.
| Case | Selected clinical and laboratory features | Histopathological features on biopsy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (1) |
Sex | Associated conditions (2) |
Positive autoantibodies (3) |
Negative autontibodies (3) |
Duodenum (4) |
Esophagus (5) |
Stomach (5) |
Ileum (5) |
Jejunum (5) |
Colon (5) |
Absent cells |
|
| 1 | 2 mo | F | Response to tx |
ACD | N | CI | N | |||||
| 2 | 1 | M | IPEX | ACD | IELs, EoE | CI | N | N | ||||
| 3 | 11 | M | Anti- Enterocyte, RF, PC |
TTG IgA, ANA, Ro, La, Sm, RNP, Jo-1, Scl- 70, dsDNA |
ACD | EoE | ACI, apop, microab, MNGC |
CI | N | |||
| 4 | 3 mo | M | Response to tx |
ACD | N | AI | N | |||||
| 5 | 5 mo | M | Netherton′s syndrome |
ACD | N | CI | N | N | ||||
| 6 | 6 mo | M | IPEX | ANA, Ro, La, Sm, RNP, Jo-1, Scl-70, dsDNA, RF, anti- thyroid antibodies, anti- pancreatic islet cell antibodies |
ACD | EoE | CI, apop | ACI | ||||
| 7 | 10 | M | SCID, HG | ANA, LKM, SMA |
ACD | N | CI | IELs | AI, microab |
|||
| 8 | 49 | F | Anti- Enterocyte, ANA, PC |
TTG IgA, TTG IgG, anti- phospoholipid |
ACD | N | AIG | ACI | IELs, apop | |||
| 9 | 65 | F | HT | ANA, AMA, AGA IgG |
Anti-Enterocyte, AGA IgA, TTG IgA, TTG IgG, RF, CCP IgG, dsDNA |
ACD | AI | CI, apop | ACI | ACI, MNGC |
||
| 10 | 2 mo | M | Anti-Enterocyte | TTG IgA, TTG IgG, TTG IgM, endomysial IgA |
ACD | EoE | CI | AI, microab |
||||
| 11 | 80 | F | HT | ANA | TTG IgA, TTG IgG | ACD | CI | ACI, apop | ||||
| 12 | 74 | F | Response to tx |
TTG | ACD | ACI, apop | ACI | |||||
| 13 | 62 | F | Uveitis, arthritis |
ANA, SMA | ANCA, AMA, TTG IgA, TTG IgG, RF, CCP, dsDNA, Ro, La, Sm, RNP |
ACD* | ACI, apop |
ACI, apop | ||||
| 14 | 6 | M | ANA, ANCA | TTG, SMA, LKM |
Celiac-like | N | CI | ACI, microab |
N | |||
| 15 | 3 | F | AIH, diabetes, JRA |
ANA, LKM, PC, GAD |
TTG, RF, Lupus screen, complement screen, thyroid antibody screen |
Celiac-like | ||||||
| 16 | 1 | M | Response to tx |
TTG | Celiac-like | N | N | IELs | ||||
| 17 | 8 mo | M | Anti-Enterocyte | TTG IgA, endomysial IgA, SMA |
Celiac-like | N | CI | ACI | ||||
| 18 | 72 | M | DM1 | ANA, CCP | TTG IgA, AGA, gliadin IgG, Ro, La, Sm, RNP, Jo-1, Scl-70, RF |
Celiac-like | ACI | Apop | Paneth | |||
| 19 | 1 | M | Response to tx |
GvHD-like | N | Apop | N | |||||
| 20 | 10 | F | HG, JRA, AIH |
ANA | GvHD-like | N | Apop | Apop | Apop | Paneth, Goblet |
||
| 21 | 26 | F | Pan- endocrine autoimmune disease (thyroid, adrenal, hepatic) |
ANA, ANCA, TTG, thyroid antibody screen, adrenal antibody screen |
GvHD-like | ACI, apop | ACI, apop | |||||
| 22 | 2 | F | HG | GvHD-like | N | N | Apop | |||||
| 23 | 4 | M | HT, congenital syndromes, Sotos syndrome |
ANA, Ro, La, Sm, RNP, Jo-1, Scl-70, dsDNA |
Mixed | N | N | N | ACI, IELs, apop, MNGC |
|||
| 24 | 2 | F | Probable DNA ligase 4 deficiency, HA, HT, IPEX mutation negative |
ANA, AGA | Mixed | N | CI | ACI | N | |||
| 25 | 63 | M | Response to tx |
ANA, TTG | Mixed | IELs | ACI, apop | Goblet | ||||
Age at the time of diagnosis, in years, unless specified as mo (months).
AIH: autoimmune hepatitis; HA: Coombs positive hemolytic anemia; DM1: type 1 diabetes mellitus; HG: hypogammaglobulinemia; HT: hypothyroidism; IPEX: Immune dysregulation, Polyendocrinopathy, Enteropathy, and X-linked syndrome; JRA: juvenile rheumatoid arthritis; Response to tx: clinical response to immunosuppressive therapy; SCID: Severe Combined Immunodeficiency
AGA: antigliadin antibody; AMA: anti-mitochondrial antibody; ANA: antinuclear antibody; ANCA: anti-neutrophil cytoplasmic antibody; CCP: anti-cyclic citrullinated peptide antibody; dsDNA: anti-double stranded DNA antibodies; GAD: anti-glutamic acid decarboxylase antibody; Jo-1: anti-Jo-1 antibody; La: anti-La/SSB antibody; LKM: anti-liver kidney microsomal antibody; PC: anti-parietal cell antibody; RF: rheumatoid factor; RNP: anti-ribonucleoprotein antibody; Ro: anti-Ro/SSA antibody; Scl-70: anti-Scl-70 (anti-topoisomerase I) antibody; Sm: anti-Smith antibody; SMA: anti-smooth muscle antibody; TTG: tissue trans-glutaminase antibody.
ACD: active chronic duodenitis pattern; Celiac-like: celiac disease-like pattern; GvHD-like: acute Graft-versus-Host Disease-like pattern; Mixed: mixed or no predominant pattern. See text for detailed explanation.
ACI: acute and chronic inflammation; AI: acute inflammation; AIG: chronic atrophic gastritis with features of autoimmune gastritis; apop: increased gland/crypt epithelial apoptosis; CI: chronic inflammation; EoE: >20 intraepithelial eosinophils per high-power field; IELs: increased intraepithelial lymphocytes; microab: neutrophilic gland/crypt microabscesses; MNGC: multinucleated epithelioid giant cells; N: no histopathological abnormalities. See text for detailed explanation.
Classified as ACD pattern on the basis of ileal biopsy (a duodenal biopsy was not performed).
Active chronic duodenitis (ACD)
Active chronic duodenitis (ACD) represented the most common pattern of injury, noted in 13 of 25 cases (52%). This pattern is characterized by moderate to severe villous blunting, expansion of the lamina propria by an inflammatory infiltrate that is predominantly mononuclear (comprised by plasma cells and lymphocytes) but includes scattered eosinophils and neutrophils, and neutrophilic cryptitis, with or without neutrophilic crypt abscesses (Fig. 1). Note that duodenal biopsy was not performed in one case (case 13), but ileal biopsy exhibited findings indistinguishable from the ACD pattern of injury. The case was therefore classified as ACD on the basis of ileal biopsy (see below for detailed discussion of ileal biopsy findings).
Figure 1. Active chronic duodenitis.
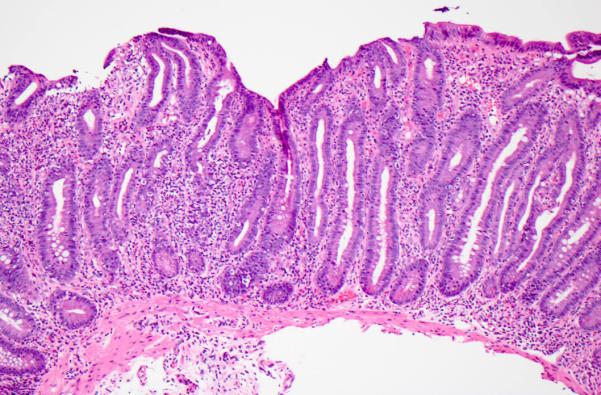
This pattern is characterized by moderate to severe villous blunting, expansion of the lamina propria by a mixed but predominantly mononuclear inflammatory infiltrate, and neutrophilic cryptitis; crypt epithelial apoptosis may be increased. Hematoxylin and eosin.
Crypt abscesses were identified in 3 cases (23%). Severe villous blunting was common, as it was seen in 10 cases (77%). The remaining 3 cases exhibited moderate villous blunting. Intraepithelial lymphocytes were elevated in only 2 of 13 cases (15%). Crypt epithelial apoptosis was increased in 6 cases (46%). The ACD pattern was the most common pattern seen in both pediatric patients (47%) and in adults (63%). It was also the most common pattern seen in patients with positive anti-enterocyte antibodies (3 of 4, 75%) (Fig. 2).
Figure 2. Neutrophilic crypt microabscess in active chronic duodenitis.
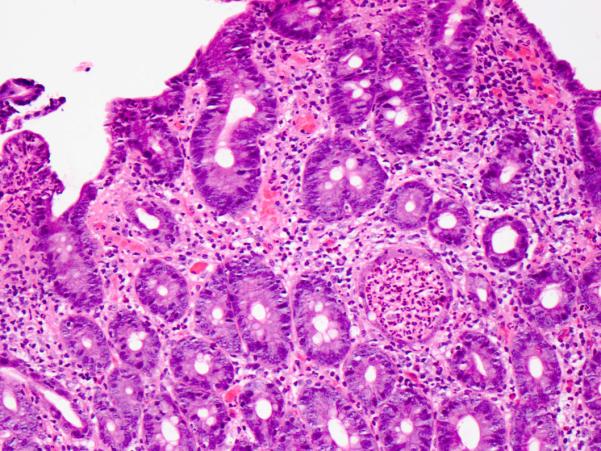
Hematoxylin and eosin.
Celiac disease-like (Celiac-like)
The second most common pattern observed was Celiac-like (5 cases, 21%). These cases exhibited villous blunting with crypt hyperplasia and markedly increased intraepithelial lymphocytes in surface epithelium (>40 IELs/100 enterocytes). The degree of villous blunting was less prominent than that seen in ACD: it was mild in 3 cases (60%), moderate in 1 case (20%), and severe in 1 case (20%). Increased crypt epithelial apoptosis was seen in 3 cases (60%). Neutrophilic inflammation was absent in all 5 cases. Testing for celiac disease serology was performed in all 5 cases, and was negative (Table 1). In addition, HLA DQ2/8 testing was performed in 2 cases (cases 14 and 16), and was negative (Fig. 3).
Figure 3. Celiac disease-like pattern.
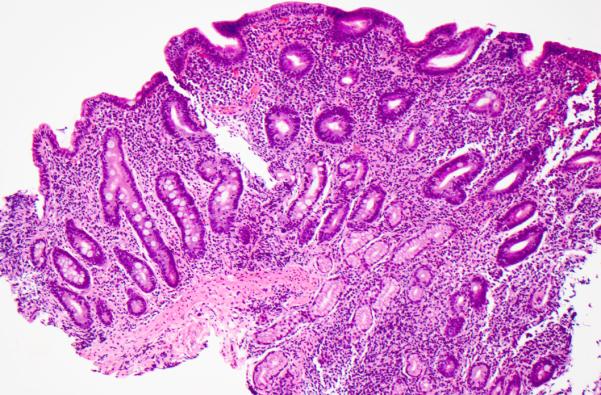
This pattern is largely indistinguishable from celiac disease, and is characterized by villous blunting and increased intraepithelial lymphocytes in surface epithelium. Hematoxylin and eosin.
Acute Graft-versus-Host Disease-like (GvHD-like)
GvHD-like pattern was observed in 4 cases (17%). These cases exhibited villous blunting with prominent crypt epithelial cell apoptosis and relative absence of inflammation (Fig 4). Interestingly, although single cell apoptosis was prominently increased (Fig 5), crypt dropout was not observed, suggesting that the histopathologic features are not entirely analogous to acute GvHD. Despite the absence of crypt dropout, significant villous blunting was observed (moderate in 3 cases and severe in 1 case).
Figure 4. Graft-versus-Host Disease-like pattern.
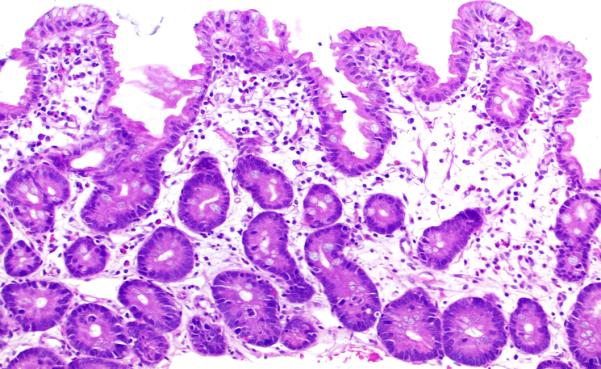
This pattern is characterized by villous blunting with increased crypt epithelial apoptosis and minimal inflammation, mimicking acute Graft-versus-Host Disease. Hematoxylin and eosin.
Figure 5. Increased crypt epithelial apoptosis in Graft-versus-Host Disease-like pattern.
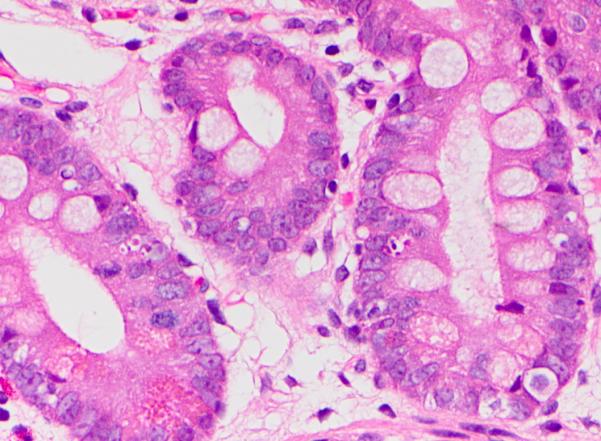
Hematoxylin and eosin.
Mixed/no predominant pattern
3 cases (13%) exhibited findings that would not allow classification into any of the above 3 patterns of injury. These cases exhibited villous blunting and expansion of the lamina propria by a mixed, predominantly mononuclear inflammatory infiltrate (comprised of plasma cells, lymphocytes, and admixed eosinophils) (Fig. 6). Increased crypt epithelial apoptosis was present in 2 cases (66%), but the amount of inflammation did not allow for their classification as GvHD-like. The degree of villous blunting was moderate in all 3 cases (100%). Neutrophilic inflammation was absent.
Figure 6. Mixed/no predominant pattern.

This pattern consists of features that do not allow classification into the previous 3 patterns, and typically exhibits villous blunting with a mixed, predominantly mononuclear inflammatory infiltrate in the lamina propria. Hematoxylin and eosin.
Histopathologic findings in the jejunum and ileum
Jejunal biopsies (performed via push enteroscopy) were available in 2 cases. In both cases, the jejunum showed an active chronic jejunitis pattern of injury, essentially identical to that seen in the corresponding biopsy of the duodenum.
Ileal biopsies were available in 12 cases, and were abnormal in 9 cases (75%). In 5 cases (42%) the ileum exhibited a pattern of injury identical to ACD. Thus, active chronic ileitis was also the most common pattern of injury observed in the ileum. Of these 5 cases, a duodenal biopsy was not available in 1 case (case 13); 2 cases exhibited ACD in the duodenum; and 2 cases exhibited different patterns of injury in the duodenum (1 case of Celiac-like and 1 case of Mixed). Severe villous blunting was seen in 3 of the 5 cases, neutrophilic crypt microabscesses in 1, and increased crypt epithelial apoptosis in 2. Pseudopyloric gland metaplasia was not observed in any cases. If we collectively consider all small intestinal sites (duodenum, jejunum, and ileum), the active chronic enteritis pattern of injury was seen in at least one small intestinal site in 15 of 25 patients (60%). The remaining 4 abnormal ileal biopsies comprised 2 cases (17%) of increased intraepithelial lymphocytes, with or without expansion of the lamina propria; 1 case (8%) of increased apoptosis in crypt epithelium; and 1 case (8%) with mild villous blunting and expansion of the lamina propria, but no active inflammation or increased intraepithelial lymphocytes.
Absence of an epithelial cell subtype in the small intestine was observed in only 3 of the 25 cases (12%): one case with absence of Paneth cells, one with absence of Goblet cells, and one with absence of Paneth and Goblet cells.
Histopathologic findings in other sites in the tubular gut
Biopsies from sites other than the small intestine (esophagus, stomach, or colon) were available for 24 of 25 patients (96%). Abnormal histopathologic findings in non-small intestinal biopsies were present in all 24 patients (100%). A single non-small intestinal site was abnormal in 13 patients (54%), and 2 or more were abnormal in 11 patients (46%). The findings in these sites were variable and cases were not easily classified into mutually exclusive patterns as was the case in the small intestine.
Stomach
The gastric mucosa was the most common non-small intestinal site to exhibit abnormalities (19 of 22 cases, 86%). Expansion of the lamina propria by mononuclear inflammation, consisting of lymphocytes and plasma cells, without activity (nonspecific chronic gastritis) was seen in 11 cases (50%). Active chronic gastritis (mononuclear inflammation in the lamina propria with neutrophilic glanditis) (Fig. 7) was present in 4 cases (18%), 1 of which exhibited neutrophilic glandular microabscesses. Active gastritis alone was present in 1 case (5%). One case (5%) showed chronic atrophic gastritis in the body/fundus with loss of parietal cells and ECL cell hyperplasia (confirmed by immunohistochemical stains for gastrin and chromogranin). Although intestinal metaplasia was not identified, the histopathologic features were essentially those of autoimmune atrophic gastritis. The patient had iron-deficiency anemia at presentation and had a positive anti-parietal cell antibody.
Figure 7. Active chronic gastritis involving the gastric antrum.
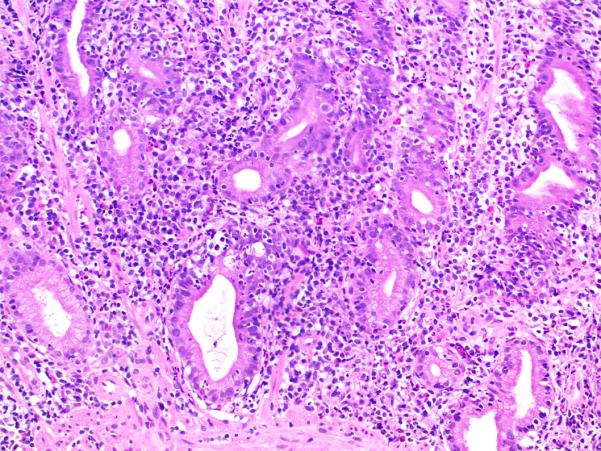
Hematoxylin and eosin.
Increased apoptosis in glandular epithelium (with or without inflammation) was present in 7 cases (32%); of these, 2 cases showed atrophic glands with luminal debris (Fig. 8). In 3 of these 7 cases the duodenum exhibited a GvHD-like pattern, while in the remaining 3 cases, it showed ACD. Epithelioid multinucleated giant cells were present in 1 case (5%). Immunohistochemical stains for Helicobacter pylori were available in 6 cases, and were all negative.
Figure 8. Atrophic gland with luminal debris in the gastric body/fundus.
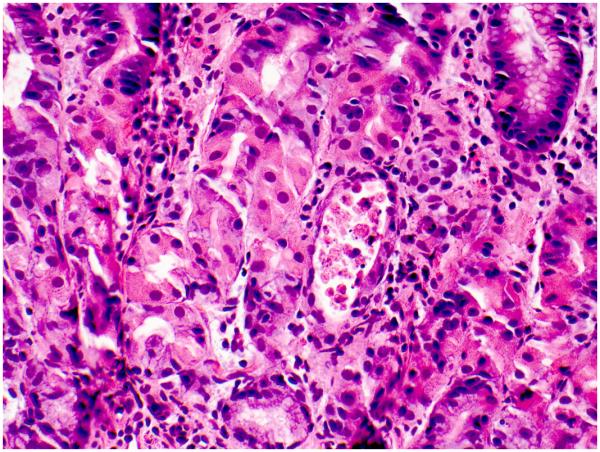
This pattern of gastric involvement mimics acute Graft-versus-Host Disease. Hematoxylin and eosin.
Colon
The colonic mucosa was abnormal in 14 of 22 cases with available biopsies (64%). Active chronic inflammation, characterized by expansion of the lamina propria by a mononuclear inflammatory infiltrate (consisting of lymphocytes and plasma cells) and neutrophilic cryptitis (Fig. 9), was present in 7 cases (32%). Active inflammation alone was seen in 2 cases (9%). Neutrophilic crypt microabscesses were seen in 2 cases (9%). Increased intraepithelial lymphocytes, with or without expansion of the lamina propria, reminiscent of lymphocytic colitis, were seen in 3 cases (14%). Increased crypt epithelial cell apoptosis (with or without inflammation) was seen in 8 cases (36%) (Fig. 10), including 1 case with atrophic crypts with luminal debris (5%). Multinucleated giant cells were seen in 2 cases (9%). Crypt architectural distortion with crypt dropout was noted in 2 cases (9%). Two cases (9%) exhibited Paneth cell metaplasia in the distal colon.
Figure 9. Colitis with lymphoplasmacytic expansion of the lamina propria and neutrophilic cryptitis.
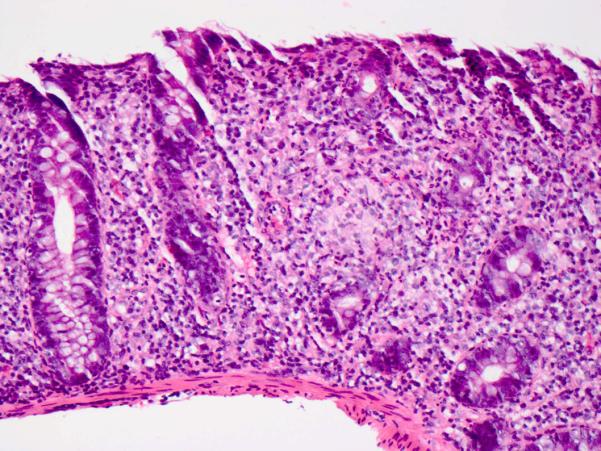
Hematoxylin and eosin.
Figure 10. Colon with strikingly increased apoptosis in crypt epithelium.
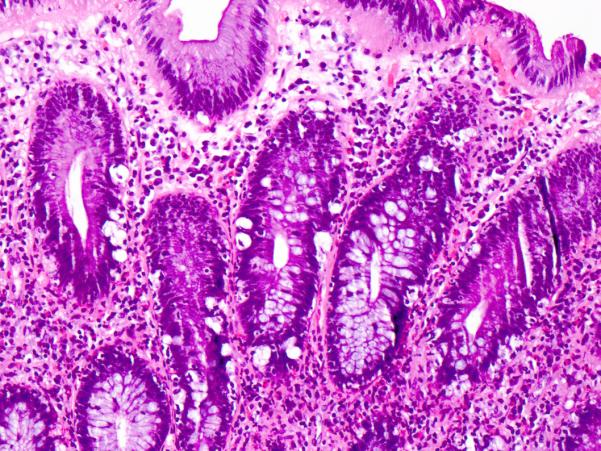
This pattern of colonic involvement mimics acute Graft-versus-Host Disease. Hematoxylin and eosin.
The findings in the colon did not necessarily coincide with the pattern of injury observed in the small intestine. Of the 3 patients with increased intraepithelial lymphocytes in the colon, only 1 had duodenal biopsies with Celiac-like pattern, and of the 8 patients with increased apoptosis in the colon, only 3 had GvHD-like pattern in the duodenum.
Esophagus
The esophageal mucosa was abnormal in 5 of 18 cases with available biopsies (28%). Eosinophilic infiltration of squamous epithelium was the most common findings, seen in 4 cases (22%). The peak intraepithelial eosinophil count exceeded 40 per high-power field in 2 of the 4 cases. In one of the 4 cases, biopsies at the time of presentation showed increased intraepithelial lymphocytes (reminiscent of so-called lymphocytic esophagitis), and subsequent biopsies showed eosinophilic infiltration. Neutrophilic inflammation was present in 1 case (6%). Occasionally, apoptotic keratinocytes were observed at the base of the squamous mucosa.
Treatment and follow-up
A detailed treatment history was available for 17 patients. 16 patients (94%) received steroids (prednisone, prednisolone, methylprednisolone, or budesonide), 9 patients (53%) received immunomodulators (azathioprine, methotrexate, or cyclosporine), 7 patients (41%) received the anti-T-cell agent tacrolimus, and 6 patients received the anti-TNF agent infliximab (35%). 2 patients (13%) had a bone marrow transplant because of debilitating clinical symptoms and failure of immunosuppressive therapy. Biopsies performed one month post-transplant in one patient showed near complete resolution of histological changes.
Clinical follow-up was available for 12 patients, with a mean length of follow-up of 47.2 months after diagnosis, and a median of 27.5 months after diagnosis. 2 patients (17%) achieved complete clinical remission, 7 patients (58%) achieved partial response, and 3 patients (25%) achieved no or minimal response; all 3 died. Follow-up biopsies after treatment were available for 19 patients. In patients with multiple sets of follow-up biopsies, only the most recent follow-up biopsy was considered for the purposes of outcome analysis. The mean length of follow-up was 36.8 months after diagnosis, and the median was 25 months after diagnosis. 9 patients (47%) achieved complete histological remission (no histopathological abnormalities observed). 5 patients (26%) experienced partial histological response, as their follow-up biopsies remained abnormal but significantly improved with respect to biopsies at the time of diagnosis. 5 patients (26%) experienced no or minimal histological response, with follow-up biopsies essentially unchanged.
In the 7 cases where both clinical and histological follow-up was available, we did not observe a consistent correlation between the two. Clinical and histological response (whether partial or complete) was seen in 4 of 7 cases (57%). In 2 cases (29%), there was no or minimal histological response but partial or complete clinical response, while in 1 case (14%) there was partial histological response but no clinical response.
DISCUSSION
We present here the largest series of AIE reported to date, including both pediatric and adult patients, and syndromic and non-syndromic patients. Our two key observations are that the most common pattern of injury on small intestinal biopsy is an active chronic enteritis (ACE) with moderate to severe villous blunting and prominent neutrophilic inflammation; and that histopathologic abnormalities at other sites in the GI tract are exceedingly common. Awareness of these two aspects of AIE should help the pathologist recognize an entity which lacks truly specific histopathologic features.
AIE commonly presents with acute and chronic inflammation in the small intestine
Active chronic enteritis was seen in 50% of duodenal biopsies. We chose to center our study on the duodenum, given that this site is the most likely to be biopsied at the time of presentation, and has received the most attention in the literature. However, we found that involvement of the ileum by AIE (regardless of the pattern of injury) was very common. If we consider duodenal and ileal biopsies together, the active chronic enteritis pattern of injury becomes even more common, as it was seen in at least one site in 60% of cases. We use the descriptor “chronic” in its broad sense, meaning expansion of the lamina propria by a mixed inflammatory infiltrate containing mononuclear cells (lymphocytes and plasma cells) and eosinophils, rather than chronicity of inflammatory bowel disease (IBD) type.
Our findings are in general agreement with three previous studies: a series of 12 IPEX syndrome patients reported by Patey-Mariaud de Serre and coworkers(9), a series of 15 adult AIE patients reported by Akram and coworkers(4), and a series of 14 pediatric AIE patients reported by Singhi and co-workers(13). With some differences in emphasis, they describe a comparable pattern of injury in 9 of 12 (75%) cases of IPEX syndrome, 8 of 14 cases (57%) of adult AIE, and 11 of 14 cases (79%) of pediatric AIE(4, 9, 13). Akram and coworkers describe the pattern as “increased mononuclear inflammation in the lamina propria, lymphocytic infiltration into deep crypt epithelium with a relative paucity of surface lymphocytosis (<40 lymphocytes per 100 epithelial cells), and increased numbers of crypt apoptotic bodies”; neutrophilic cryptitis and villitis was seen in 5 of the 8 cases(4). Singhi and co-workers describe the pattern as “villous blunting, crypt hyperplasia, lymphoplasmacytic expansion of the lamina propria and crypt apoptosis”, and add that neutrophilic cryptitis was seen in 4 of the 11 cases (including neutrophilic crypt microabscesses in 3 cases)(13). Patey-Mariaud de Serre and coworkers refer to the pattern as “GvHD-like pattern” to emphasize increased apoptosis; the remaining features of the pattern, however, are more in keeping with our pattern of acute chronic enteritis: severe villous atrophy, moderate-to-marked mixed inflammation in the lamina propria (composed of lymphocytes, plasma cells, neutrophils, and eosinophils), and neutrophilic crypt abscesses(9). In our series, the inflammatory changes were more characteristic than increased apoptosis, which was prominent in only a minority of cases (8 of 25, 32%). We thus chose to designate “GvHD-like” those cases with prominent apoptosis but minimal inflammatory changes, which are a more adequate comparison to actual acute GvHD.
Absence of an epithelial cell subtype (Paneth cells or goblet cells) has been reported in a number of AIE cases (17-19), but we found this to be uncommon (12%). Absence of an epithelial cell subtype varies widely in case series, from 8% to 60%(4, 9, 13). Thus, although this feature is certainly helpful when present, in practical terms we believe that one cannot rely on it to suggest the diagnosis of AIE.
We found that normalization of small intestinal histology after treatment is common, although biopsy findings may not correlate completely with the clinical course. Histological resolution with therapy has been previously reported(4), and histologic improvement in the absence of clinical improvement (which we observed in 1 case) has also been noted(17). Although no specific recommendations exist regarding repeat endoscopy with biopsy in the management of AIE patients, our experience is that clinicians will often perform it (in some patients, on an annual basis) and request comparison to the most recent prior biopsy in order to gauge histological improvement.
Celiac disease remains an important differential diagnostic consideration
In a subset of AIE patients, small intestinal biopsies exhibited changes essentially indistinguishable from celiac disease, with villous blunting and markedly increased intraepithelial lymphocytes in surface epithelium. This is recognized in the literature(4, 9, 13, 20-22) and represents a significant diagnostic challenge. In all 5 cases we report with this histopathological pattern, serological studies for celiac disease were negative, which allowed exclusion of celiac disease (HLA DQ2/8 testing was performed in 2 cases and was also negative). However, celiac serology may be positive in AIE patients(4, 13). We observed positive antigliadin antibodies in 2 of 25 patients, but neither of these patients exhibited celiac disease-like histology. Similarly, even when duodenal biopsies show villous blunting and increased intraepithelial lymphocytes, the presence of other histological features of AIE (prominent apoptosis and absence of epithelial cell subtypes) may facilitate recognition of AIE in patients with positive celiac serology(13).
The most challenging scenario is the small subset of AIE patients with both celiac disease-like histology on small intestinal biopsy and positive celiac serology (2 out of 15 in one study)(4). These patients will not respond to gluten-free diet and will need immunosuppressive therapy. Recognition of AIE in this setting necessitates consideration of other clinical and laboratory features. We find biopsies of non-small intestinal sites to be helpful in this scenario. In 3 of the 5 patients with Celiac-like duodenal histology, biopsies outside the small intestine showed abnormalities that would be considered unusual for celiac disease (acute and chronic inflammation in stomach, ileum, or colon; and increased apoptosis in the colon). We recognize that, in particularly difficult instances, the distinction between AIE and celiac disease may necessitate exclusion of HLA DQ2/8 phenotypes(23). In cases with a more mixed histopathological picture, we find the extent of neutrophilic inflammation, and in particular the presence of crypt microabscesses, to be helpful in distinguishing AIE from celiac disease. Although neutrophilic inflammation may be seen in celiac disease, crypt microabscesses are exceedingly rare, as they are present in less than 1% of cases(24).
Occasionally, biopsies from the tubular gut exhibited features that may raise the possibility of IBD; specifically, Crohn’s disease. We observed multinucleated epitheloid giant cells in 3 cases (2 in the colon, 1 in the stomach), but no well-formed granulomas were seen. Crypt architectural distortion was observed in the colon in 2 cases; however, basal lymphoplasmacytosis was absent. Crypt architectural changes were not seen in the small intestine, and pseudopyloric gland metaplasia was not seen in the ileum. Paneth cell metaplasia in the distal colon was seen in 2 cases. Although Paneth cell metaplasia is considered an indication of chronic mucosal injury, it is by no means specific to IBD, as it is recognized to occur in other gastrointestinal diseases such as lymphocytic colitis, collagenous colitis, and cord colitis syndrome(25-27). We believe that the overall histopathologic features, in conjunction with the clinical presentation, should direct the pathologist away from strongly considering Crohn’s disease. From a purely pathologic standpoint, the differential diagnosis of the active chronic enteritis pattern is broad, and includes infection, drug-mediated injury (particularly olmesartan(28)), and acute GvHD. Correlation with clinical and laboratory parameters is of critical importance for the pathologist to arrive at the correct diagnosis.
Histopathologic abnormalities outside the small intestine are seen in all AIE patients
We found abnormalities in non-small intestinal sites to be the norm, although protean in nature. This is overall consistent with previous reports and lends support to the view that AIE is a disease of the entire gastrointestinal tract(7, 13, 29). Reported findings in the stomach and colon comprise a spectrum similar to what we observed, and include acute inflammation, chronic inflammation, increased apoptosis, and lymphocytic infiltration of epithelium(4, 7, 9, 13, 17, 21, 29, 30). Chronic atrophic gastritis involving the body/fundus with features of autoimmune gastritis, as we observed in 1 case, has been previously reported in AIE patients(4, 13). We thus recommend upper and lower endoscopy with biopsies when AIE is in the differential diagnosis clinically or pathologically. As noted above, this may help distinguish AIE from celiac disease. In one case (case 13), colonic biopsies performed 15 months prior to the diagnosis of AIE showed lymphocytic colitis. The patient presented with diarrhea and was treated with budesonide without good response. Since small intestinal biopsies were not performed when the patient presented with lymphocytic colitis, we do not know whether there was histological evidence of enteropathy at the time. However, this raises the possibility that lymphocytic colitis preceded development of AIE, which to our knowledge has not been previously reported.
The presence of eosinophilic inflammation in esophageal biopsies may represent superimposed eosinophilic esophagitis, rather than a manifestation of AIE. Involvement of the esophagus by AIE appears to be less common than the stomach or colon, but is recognized to occur(17, 30). However, we could not identify features that would allow distinction of these cases from eosinophilic esophagitis.
The significance of autoantibodies to gut epithelial cells
The pathogenic significance and diagnostic relevance of circulating autoantibodies to gut epithelial cells (anti-enterocyte and anti-goblet cell antibodies) remains controversial. In our series, anti-enterocyte antibodies were detected in 4 of 5 patients tested (80%). Detection of said antibodies has been proposed as a diagnostic criterion for AIE by Akram and coworkers, who detected anti-goblet cell or anti-enterocyte antibodies in 13 of 14 patients in their adult series(4). Similarly, Patey-Mariaud de Serre and coworkers detected anti-enterocyte antibodies in all 12 patients in their IPEX syndrome series(9). However, patients with clinical and pathological features otherwise characteristic of AIE may lack these antibodies(21). This includes patients in the series reported by Akram and coworkers(4) and the original series reported by Unsworth and Walker-Smith, who therefore accepted either circulating anti-enterocyte autoantibodies or presence of other autoimmune diseases as proof of autoimmunity for diagnostic purposes(1). In the series by Singhi and coworkers, anti-enterocyte antibodies were positive in 10 of 14 patients (71%) at presentation(13).
More importantly, the specificity of anti-enterocyte and anti-goblet cell antibodies for AIE remains in question, as their rate of detection in non-AIE patients varies widely in the literature. Some studies have shown that they are not detected in patients with various gastrointestinal illnesses other than AIE(1, 7, 20, 31). Others, however, have detected them in patients with a wide range of gastrointestinal ailments (including IBD, celiac disease, cows’ milk insensitivity, and protein-losing enteropathy) and with other conditions such as HIV(8, 10, 11, 32, 33). For example, in one study, antibodies to intestinal epithelial cells were detected in 69.7% of IBD patients and 55.7% of their relatives(11). In AIE patients, testing for anti-enterocyte antibodies may be negative at presentation but become positive afterwards(13). Some authors therefore consider these antibodies to be non-specific indicators of mucosal injury(3), and recommend that they not be used as a diagnostic criterion(13).
In view of this, we believe that it is not possible to rely on antibody testing for the diagnosis of AIE. From a practical standpoint, further difficulty arises from the fact that such testing is not standardized, its interpretation is observer-dependent, and it is not readily available at most institutions. For example, at one institution in this study (MGH), testing is available for anti-enterocyte antibody only, and is performed in a research laboratory. Even if widespread consensus on the role of antibody testing is achieved, we believe the diagnosis of AIE is likely to remain a clinicopathologic correlation. In our study, we have followed the diagnostic criteria for AIE originally proposed by Unsworth and Walker-Smith(1)(see Methods). Essentially the same criteria were used by both Akram and coworkers(4) and Singhi and coworkers(13) to select patients for inclusion in their series (with the added requirement of adult onset in the former): severe/protracted diarrhea unresponsive to dietary exclusion, associated with villous atrophy on small intestinal biopsy, and with gut epithelial cell autoantibodies and/or evidence of autoimmunity. The fulfillment of the last criterion is variable, particularly given the lack of consensus regarding the utility of gut epithelial cell autoantibodies. Like Singhi and coworkers, we have accepted non-gut epithelial cell autoantibodies, other autoimmune diseases, or clinical response to immunosuppressive therapy as evidence of autoimmunity(13). In the series by Akram and coworkers, if gut epithelial cell autoantibodies are not considered, a predisposition to autoimmunity on the basis of positive circulating autoantibodies or associated autoimmune disorders was seen in 12 of 15 patients (80%)(4).
In summary, we find that AIE most commonly presents in the small intestine with villous blunting, lamina propria expansion by mixed but predominantly mononuclear inflammation (consisting of plasma cells and lymphocytes), and neutrophilic cryptitis. Increased apoptosis is not always seen but is a helpful feature when present. There is a comparatively minor increase in intraepithelial lymphocytes, although a minority of cases is largely indistinguishable from celiac disease. Histopathologic abnormalities outside the small intestine are exceedingly common and may facilitate recognition of this uncommon disease by the pathologist. AIE appears to be a pan-gastrointestinal disorder and further research is needed to understand its pathogenesis.
Acknowledgments
Ricard Masia is supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA09216.
Footnotes
Disclosures: The authors have no conflicts of interest.
REFERENCES
- 1.Unsworth DJ, Walker-Smith JA. Autoimmunity in diarrhoeal disease. J Pediatr Gastroenterol Nutr. 1985;4:375–380. doi: 10.1097/00005176-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep. 2012;14:380–385. doi: 10.1007/s11894-012-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montalto M, D'Onofrio F, Santoro L, et al. Autoimmune enteropathy in children and adults. Scand J Gastroenterol. 2009;44:1029–1036. doi: 10.1080/00365520902783691. [DOI] [PubMed] [Google Scholar]
- 4.Akram S, Murray JA, Pardi DS, et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5:1282–1290. doi: 10.1016/j.cgh.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 7.Hill SM, Milla PJ, Bottazzo GF, et al. Autoimmune enteropathy and colitis: is there a generalised autoimmune gut disorder? Gut. 1991;32:36–42. doi: 10.1136/gut.32.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirakian R, Hill S, Richardson A, et al. HLA product expression and lymphocyte subpopulations in jejunum biopsies of children with idiopathic protracted diarrhoea and enterocyte autoantibodies. J Autoimmun. 1988;1:263–277. doi: 10.1016/0896-8411(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 9.Patey-Mariaud de Serre N, Canioni D, Ganousse S, et al. Digestive histopathological presentation of IPEX syndrome. Mod Pathol. 2009;22:95–102. doi: 10.1038/modpathol.2008.161. [DOI] [PubMed] [Google Scholar]
- 10.Biagi F, Bianchi PI, Trotta L, et al. Anti-goblet cell antibodies for the diagnosis of autoimmune enteropathy? Am J Gastroenterol. 2009;104:3112. doi: 10.1038/ajg.2009.511. [DOI] [PubMed] [Google Scholar]
- 11.Fiocchi C, Roche JK, Michener WM. High prevalence of antibodies to intestinal epithelial antigens in patients with inflammatory bowel disease and their relatives. Ann Intern Med. 1989;110:786–794. doi: 10.7326/0003-4819-110-10-786. [DOI] [PubMed] [Google Scholar]
- 12.Bishu S, Arsenescu V, Lee EY, et al. Autoimmune enteropathy with a CD8+ CD7− T-cell small bowel intraepithelial lymphocytosis: case report and literature review. BMC Gastroenterol. 2011;11:131. doi: 10.1186/1471-230X-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhi AD, Goyal A, Davison JM, et al. Pediatric autoimmune enteropathy: an entity frequently associated with immunodeficiency disorders. Mod Pathol. 2014;27:543–553. doi: 10.1038/modpathol.2013.150. [DOI] [PubMed] [Google Scholar]
- 14.Leon F, Olivencia P, Rodriguez-Pena R, et al. Clinical and immunological features of adult-onset generalized autoimmune gut disorder. Am J Gastroenterol. 2004;99:1563–1571. doi: 10.1111/j.1572-0241.2004.40039.x. [DOI] [PubMed] [Google Scholar]
- 15.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Washington K, Stenzel TT, Buckley RH, et al. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol. 1996;20:1240–1252. doi: 10.1097/00000478-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Moore L, Xu X, Davidson G, et al. Autoimmune enteropathy with anti-goblet cell antibodies. Hum Pathol. 1995;26:1162–1168. doi: 10.1016/0046-8177(95)90283-x. [DOI] [PubMed] [Google Scholar]
- 18.Rogahn D, Smith CP, Thomas A. Autoimmune enteropathy with goblet-cell antibodies. J R Soc Med. 1999;92:311–312. doi: 10.1177/014107689909200615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Khalidi H, Kandel G, Streutker CJ. Enteropathy with loss of enteroendocrine and paneth cells in a patient with immune dysregulation: a case of adult autoimmune enteropathy. Hum Pathol. 2006;37:373–376. doi: 10.1016/j.humpath.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Carroccio A, Volta U, Di Prima L, et al. Autoimmune enteropathy and colitis in an adult patient. Dig Dis Sci. 2003;48:1600–1606. doi: 10.1023/a:1024705032326. [DOI] [PubMed] [Google Scholar]
- 21.Casis B, Fernandez-Vazquez I, Barnardos E, et al. Autoimmune enteropathy in an adult with autoimmune multisystemic involvement. Scand J Gastroenterol. 2002;37:1012–1016. doi: 10.1080/003655202320378185. [DOI] [PubMed] [Google Scholar]
- 22.Corazza GR, Biagi F, Volta U, et al. Autoimmune enteropathy and villous atrophy in adults. Lancet. 1997;350:106–109. doi: 10.1016/S0140-6736(97)01042-8. [DOI] [PubMed] [Google Scholar]
- 23.Volta U, De Angelis GL, Granito A, et al. Autoimmune enteropathy and rheumatoid arthritis: a new association in the field of autoimmunity. Dig Liver Dis. 2006;38:926–929. doi: 10.1016/j.dld.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Moran CJ, Kolman OK, Russell GJ, et al. Neutrophilic infiltration in gluten-sensitive enteropathy is neither uncommon nor insignificant: assessment of duodenal biopsies from 267 pediatric and adult patients. Am J Surg Pathol. 2012;36:1339–1345. doi: 10.1097/PAS.0b013e318254f413. [DOI] [PubMed] [Google Scholar]
- 25.Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: histopathology and clinical course. Am J Gastroenterol. 1997;92:57–60. [PubMed] [Google Scholar]
- 26.Gupta NK, Masia R. Cord colitis syndrome: a cause of granulomatous inflammation in the upper and lower gastrointestinal tract. Am J Surg Pathol. 2013;37:1109–1113. doi: 10.1097/PAS.0b013e31828a827a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams WJ. Histology of Crohn's syndrome. Gut. 1964;5:510–516. doi: 10.1136/gut.5.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732–738. doi: 10.1016/j.mayocp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitomi H, Tanabe S, Igarashi M, et al. Autoimmune enteropathy with severe atrophic gastritis and colitis in an adult: proposal of a generalized autoimmune disorder of the alimentary tract. Scand J Gastroenterol. 1998;33:716–720. doi: 10.1080/00365529850171657. [DOI] [PubMed] [Google Scholar]
- 30.Lachaux A, Loras-Duclaux I, Bouvier R. Autoimmune enteropathy in infants. Pathological study of the disease in two familial cases. Virchows Arch. 1998;433:481–485. doi: 10.1007/s004280050277. [DOI] [PubMed] [Google Scholar]
- 31.Mirakian R, Richardson A, Milla PJ, et al. Protracted diarrhoea of infancy: evidence in support of an autoimmune variant. Br Med J (Clin Res Ed) 1986;293:1132–1136. doi: 10.1136/bmj.293.6555.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Villa JM, Camblor S, Costa R, et al. Gut epithelial cell autoantibodies in AIDS pathogenesis. Lancet. 1993;342:380. doi: 10.1016/0140-6736(93)91531-p. [DOI] [PubMed] [Google Scholar]
- 33.Skogh T, Heuman R, Tagesson C. Anti-brush border antibodies (ABBA) in Crohn's disease. J Clin Lab Immunol. 1982;9:147–150. [PubMed] [Google Scholar]


