Figure 1.
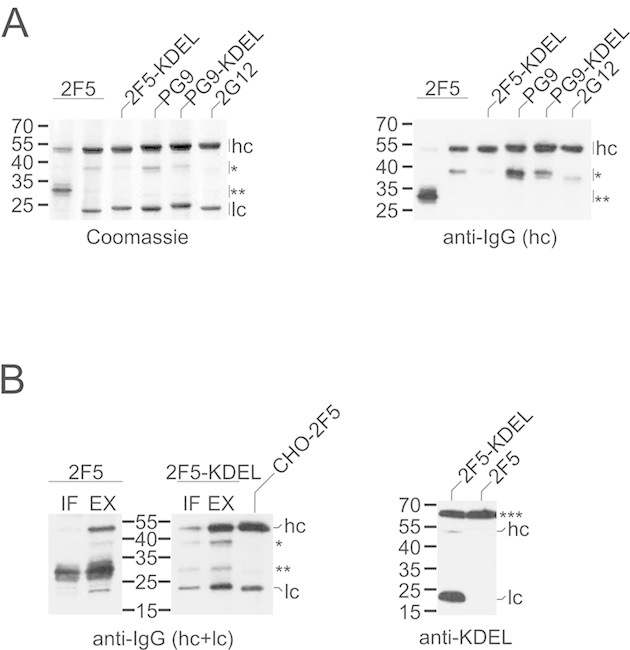
Expression of 2F5, 2G12, and PG9 in N. benthamiana. (A) mAbs were affinity-purified from total soluble leaf extracts (30 mg leaf wet weight) and then analyzed by SDS–PAGE/Coomassie blue staining (left panel) or immunoblotting with antibodies to the heavy chain of human IgG (right panel). (B) Left panel: mAbs affinity-purified from intercellular fluid (IF; 100 mg leaf wet weight) or total soluble leaf extracts prepared in the presence of proteinase inhibitors (EX; 50 mg leaf wet weight) were analyzed by immunoblotting with antibodies to the heavy and light chains of human IgG. CHO-derived 2F5 (100 ng) was loaded as control. Right panel: total soluble extracts (2 μg protein) of leaves infiltrated with different 2F5 constructs were analyzed by immunoblotting with anti-KDEL antibodies (right panel). hc, heavy chain; lc, light chain; *40-kDa fragment; **30-kDa degradation product. Endogenous N. benthamiana BiP (***) served as internal control in the case of KDEL detection. The migration positions of selected molecular mass standards are indicated, with their respective masses expressed in kDa. The results shown are representative of at least two independent experiments.
