Figure 3.
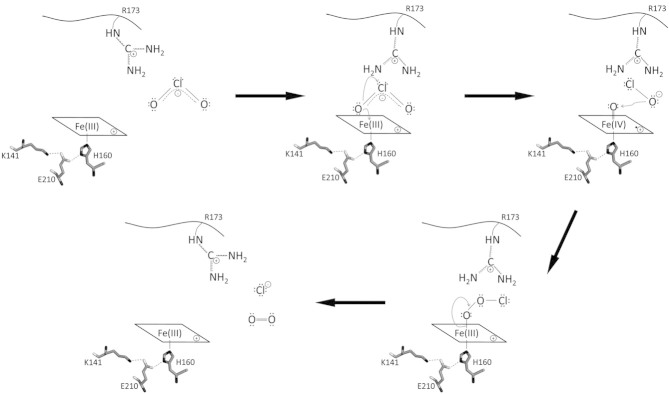
Proposed reaction mechanism of chlorite dismutase. The reaction starts with the attack of anionic chlorite at ferric heme b. After formation of the Fe(III)-chlorite complex, heterolytic cleavage of one covalent oxygen-chlorine bond in chlorite leads to the formation of hypochlorite and the redox intermediate compound I [oxoiron(IV) porphyrin cation radical]. Finally, upon nucleophilic attack of intermediate anionic hypochlorite at the ferryl oxygen, compound I is reduced to the resting state and dioxygen and chloride are released. In addition, the scheme shows the putative role of distal arginine (fully conserved in functional Clds) in the orientation and stabilization of the substrate as well as the postulated intermediate(s). On the proximal site the conserved triad H160-E210-K141 that forms a H-bonding network is depicted.
