Figure 1. Structures of WNK, OSR1, and SPAK kinases.
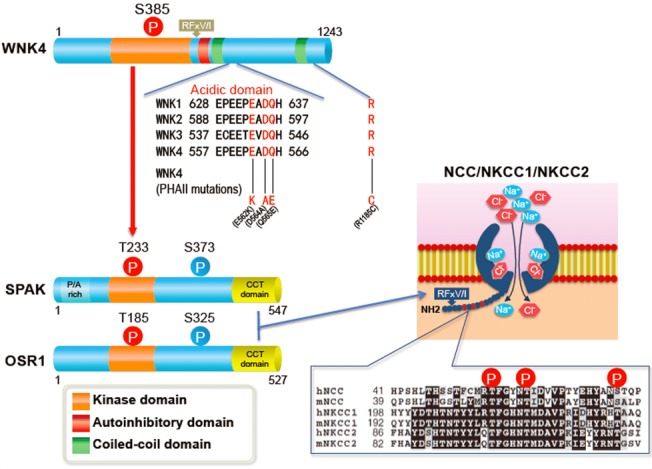
Acidic domains are located downstream of the first coiled-coil domain and conserved in all WNK kinases. Three of four pseudohypoaldosteronism type II-causing mutations in WNK4 are located in the acidic domain. WNK kinases activate OSR1 and SPAK by phosphorylating threonine residues in their kinase domains (T185 and T233). Serine residues (S325 in OSR1 and S373 in SPAK) in the S motif are also phosphorylated by WNK kinases, but their phosphorylation is not involved in the activation of the kinases. Conserved C-terminal domains in OSR1 and SPAK (shown in yellow) bind to the RFx[V/I] motif in WNK and solute carrier family 12 transporters. The N-terminal regions of NCC, NKCC1, and NKCC2 around the sites phosphorylated by OSR1 and SPAK are highly conserved.
