Figure 4. Molecular pathogenesis of pseudohypoaldosteronism type II.
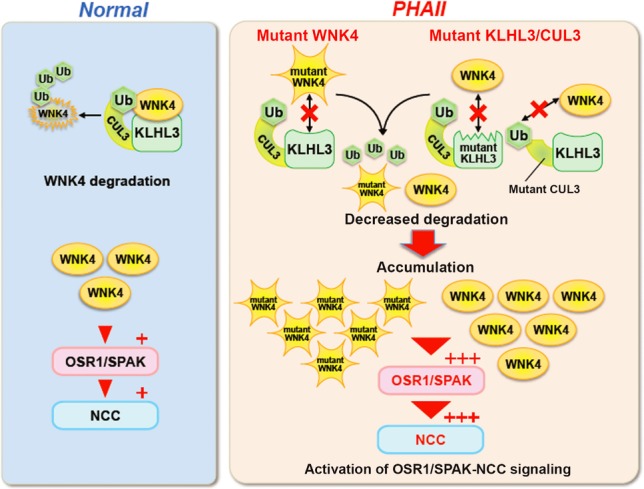
Under normal conditions, WNK 4 protein within cells are maintained by appropriate degradation after ubiquitination by KLHL3-Cullin3 E3 ligase. However, PHAII causing-mutations in the acidic domain of WNK4 and in the Kelch domains of KLHL3 affect their binding, thereby reducing the ubiquitination and degradation of WNK4. PHAII-causing mutant Cullin3 lacking the portion corresponding to exon 9 exhibits lower E3 ligase activity in combination with KLHL3 toward WNK4. Thus, PHAII-causing mutations in three different genes have a common consequence: decreased ubiquitination and increased WNK4 protein levels within cells. The increase in WNK4 protein was confirmed in the kidneys of Wnk4D561A/+ PHAII model mice. Furthermore, increased WNK4 protein levels in the kidneys of WNK4 transgenic mice activated OSR1/SPAK–NCC signaling. Although WNK4 is the major WNK kinase regulating NCC in the kidney, other WNKs normally expressed at low levels could also be increased in kidneys with PHAII caused by KLHL3 and Cullin3 mutations, thereby contributing to the more severe phenotypes resulting from these mutations compared with those resulting from WNK1 or WNK4 mutations alone.
