Figure 7. Active variants of P7C3 enhance the activity of purified NAMPT enzyme.
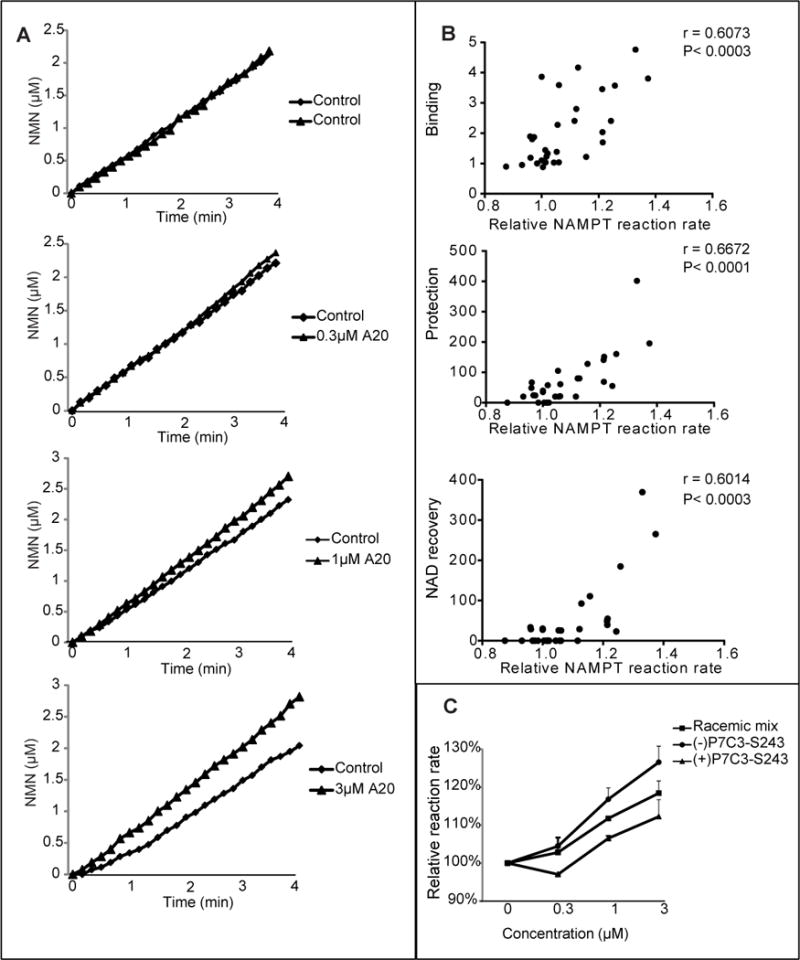
(A) P7C3-A20 was incubated at indicated concentrations in a reaction coupled with three enzymes; NAMPT, NMNAT, and ADH (Experimental Procedures). NAMPT activity was recorded for the indicated period of time at OD340nm by measuring NADH appearance, leading to the indicated concentration-time plot. Each assay was repeated three independent times with similar results. See also Figure S6. (B) Analysis of the activities of thirty P7C3 analogs was performed to assess their effects on NAMPT enzymatic activity. The reaction rate was calculated as the slope of the concentration-time curve. Relative reaction rate was normalized by the control reaction run prior to compound addition. Data represents the mean of experimental duplicates. Scatter graphs were plotted to compare the ability of compounds to activate NAMPT relative to their ability to compete away P7C3-S326 crosslinking (top scatter plot), ability to protect cells from doxorubicin-mediated toxicity (middle scatter plot), or ability to facilitate NAD restoration in doxorubicin-treated cells (bottom scatter plot). Significant correlations were observed from all three sets of data. See also Table S1. (C) Comparison of P7C3-S243 enantiomers showed that (−)-P7C3-S243 was superior to (+)-P7C3-S243 in activating the purified NAMPT enzyme. Data are expressed as mean ± SD of duplicate independent assays. See also Figure S7.
