Figure 9.
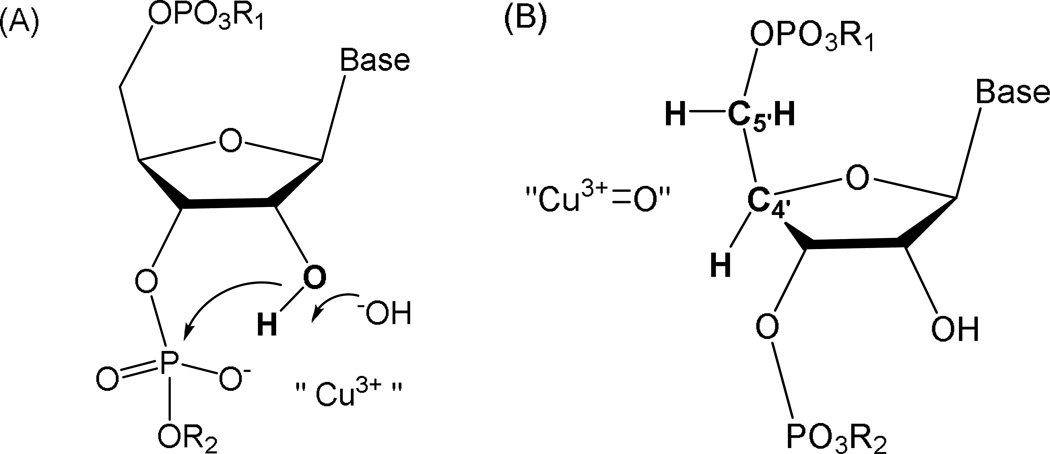
Under oxidative conditions, catalysts 1-Cu and 2-Cu can promote RNA cleavage chemistry either through “hydrolytic” (A) or “oxidative” (B) mechanisms promoted through the transient formation of higher valent Cu3+. The illustration shows initial intermediates proposed for RNA cleavage mechanisms. The requirement for redox coreagents to effect cleavage supports the requirement for Cu3+ formation and transient intermediates are shown that invoke either a role as Lewis acid in a classical hydrolysis mechanism involving a cyclic phosphate ester (A), where the Cu3+ promotes formation of an outer or inner sphere hydroxide that deprotonates the 2’-hydroxyl and/or stabilizes the build-up of negative charge density in the transition state of cyclic phosphate ester formation; or (B), by an oxidative pathway involving a copper-associated reactive oxygen species that is positioned to mediate hydrogen abstraction from the C4’ or C5’ hydrogens. For the analogous reactions for oxidative cleavage of DNA, both C4’-H or C5’-H hydrogen abstraction are most common although the oligonucleotide 3’-phosphate product can be observed for oxidative C–H scission at any ribose carbon.[29] For RNA, the oxidative reaction (B) is suggested to most likely occur by C4’-H or C5’-H hydrogen abstraction because these hydrogens are typically the most accessible and the pathways are less compromised by the presence of the C2’-OH.[28]