Abstract
To better understand if a complex process such as phagocytosis is influenced by substrate stiffness, we investigated the influence of substrate elastic modulus on phagocytosis in the retinal pigment epithelial (RPE) cell line ARPE-19. RPE cells lie on Bruch’s membrane, directly under the retina, and phagocytose the shed photoreceptor outer segments. Bruch’s membrane is known to increase in stiffness by an order of magnitude with age and thus, this study has potential relevance in explaining retinal changes in age-related macular degeneration.
ARPE-19 cells were plated on laminin-coated polyacrylamide substrates of varying elastic modulus. After 14 days in culture, a solution of latex fluorescent beads suspended in PBS was placed in each well. After an incubation time of 4 hours, flow cytometry was performed to determine the number of cells that phagocytosed a bead. The number of ARPE-19 cells that phagocytosed a bead decreased continuously as a function of increasing substrate elastic modulus (p=0.0135), and this was found to be a linear relationship (slope=−0.03305 ± 0.01104, R2 =0.4726 per 10,000 cells).
Our results suggest that RPE cells display decreased phagocytosis when grown on firmer substrates, and thus, RPE cells in older eyes, in which Bruch’s membrane is stiffer, may demonstrate decreased phagocytosis. Impaired phagocytosis by RPE cells may contribute to impaired metabolism of photoreceptor outer segments and to development of macular degeneration. Material stiffness may be a critical parameter in the development of neural therapies, including retinal prosthetics and stem cell therapies.
Keywords: Mechanotransduction, Retinal Pigment Epithelium, Phagocytosis, Flow Cytometry
Introduction
The elastic modulus of the environment in which a cell exists has recently been shown to influence cell processes such as proliferation (Janmey et al, 2009), motility (Pelham and Wang, 1998), and gene expression (Pelham and Wang, 1998) in many cell types. We sought to study the influence of substrate stiffness upon a complex cellular process and, as a model, selected phagocytosis by retinal pigment epithelial (RPE) cells.
The biomedical relevance of this research is based upon the fact that Bruch’s membrane, the basement membrane of the RPE cells, is directly under the retina and is known to increase in stiffness by an order of magnitude (from 1,000 Pa to 10,000 Pa) with age (Fischer, 1987). To better understand whether phagocytosis by RPE cells is influenced by substrate stiffness, we investigated the influence of substrate elastic modulus on phagocytosis in the RPE cell line ARPE-19.
The most common form of age-related macular degeneration is the non-exudative, or dry form. RPE cells are thought to play an important role in the development of dry macular degeneration because they are located on Bruch’s membrane, directly under the retina and provide metabolic support for the photoreceptor cells (rods and cones) (Kevany and Palczewski, 2010). As patients age, RPE cells have been found to undergo structural changes including accumulation of the pigment lipofuscin, loss of melanin, and accumulation of drusen on Bruch’s membrane (Dunn, 1996).
The process of non-exudative macular degeneration may be initiated by the failure of RPE cells to phagocytose the shed outer segments of photoreceptors (Green, 1999). This may result in an accumulation of drusen (deposits of lipids and calcium (Dunn, 1996)) adjacent to the basement membrane of RPE cells (Boulton and Wassell, 1998), atrophy of the retinal pigment layer below the retina, and loss of photoreceptors in the macula (Green, 1999), leading to vision loss.
The aim of the present study was to examine the influence of the mechanical properties of the cell culture substrate upon phagocytosis in RPE cells.
Methods and Materials
Preparation of glass coverslips
Cover slips were chemically activated to allow stable, covalent formation of polyacrylamide sheets according to the protocol of Pelham and Wang (Pelham and Wang, 1998), with modifications (Davis et al, 2012). Briefly, a glass cover slip (No. 1, 15 mm diameter; Fisher Scientific, Pittsburgh, PA) was coated with a small drop of 3-aminopropyltrimethoxysilane (TESPA, Sigma, St. Louis, MO), which was spread evenly on the surface. After 5 minutes, the coverslips were washed extensively with distilled water and then were autoclaved (121°C at 1.5 atm) for 1 hour. The coverslips were transferred, treated side up, into plastic petri dishes and covered with 0.5% glutaraldehyde in phosphate buffered saline (PBS) (prepared by diluting 1 part of 70% stock solution, Polysciences, Inc., Warrington, PA, with 140 parts of PBS). After incubation at room temperature for 30 minutes, the coverslips were washed 5 times in distilled water on a shaker, for 10 minutes per wash, and allowed to dry in air. The treated coverslips were stored in a petri dish for up to 48 hours after preparation.
Creation of Polyacrylamide Gels
Thin sheets of polyacrylamide gel were prepared and bonded to the activated glass surface of the cover slips according to Pelham and Wang (Pelham and Wang, 1998) with modifications (Davis et al, 2012). Acrylamide (Bio-Rad, Hercules, CA, 30% w/v) was mixed with N, N-methylene-bis-acrylamide (BIS, Bio-Rad, 2.5% w/v) and distilled water to obtain a final concentration of 10% acrylamide and 0.03% BIS. For more rigid or more flexible substrata the percentage of BIS was increased or decreased according to a previously established nonogram (Pelham and Wang, 1998). After the acrylamide/BIS solution was combined, polymerization was initiated by addition of ammonium persulfate (10% w/v solution, Bio-Rad, 1:200 volume) and N,N,N,N-tetramethylethylenediamine (TEMED, Bio-Rad, 1:2000 volume). Twenty-five microliters of the acrylamide solution was immediately placed onto the surface of an activated coverslip and the droplet was flattened using a large circular cover slip (No. 1, 22 mm diam., Fisher Scientific) covered in Sigma-Cote (SL-2 100ml, Sigma-Aldrich). After polymerization for 30 minutes, the circular cover slip was removed, and the gels were agitated on a shaker in 500 μl HEPES (50 mM, pH 8.5) and then transferred to a 24 well plate. The HEPES was removed from the wells and a solution of 40 μl of Sulfo-SANPAH (0.5mg in 1ml of distilled water, Pierce Chemicals, Rockford, IL) was applied to each gel. The surface of each gel was then exposed to UV light from a 30 W germicidal lamp for 10 minutes. The glass-supported polyacrylamide sheets then underwent three washes of 500 μl HEPES (50mM, pH 8.5). The polyacrylamide sheets were then covered with a 200 μl solution of laminin (1mg natural mouse laminin in 5.95ml HEPES buffer, Invitrogen™) and were incubated at 37°C for 4 hours in 5% CO2. The gels were then washed three times with 500 μl of PBS to prepare the polyacrylamide coated cover slips for cell culture. The gels were immersed in 500 μl of RPE media (225ml Dulbecco’s modified eagle medium, 25ml of fetal bovine serum, and 1% penicillin streptomycin) 15 minutes prior to applying ARPE-19 cells at 20,000 (cells/ ml) per gel. Substrates of 500 Pa, 1000 Pa, and 5000 Pa were created for this study.
Cell Culture
ARPE-19 cells (American Type Culture Collection (ATCC), Manassas, VA) were grown on the gels at 37°C for 14 days in 5% CO2, in 500 μl of RPE media and the media was changed every other day. After 14 days in culture, 35 μl of bead solution [20μl of FITC-labeled latex fluorescent beads (Polysciences Inc., diameter 1.967μm, initially packaged as a 2.5% aqueous suspension) suspended in 4ml of PBS] were added to each well, and incubated at 37°C for 4 hours in 5% CO2.
The ARPE-19 cell line is well characterized and we have previously found that the doubling time for these cells to be 60 hours. Given the plated density of cells, we thus predicted a plated cell density of 70,000 cells/well at the time of the experiments. The added solution thus had a concentration of 2.84 × 107 beads/ml and 9.94 × 106 beads total or 142 beads/cell were added to each well.
Flow Cytometry
After a 4-hour incubation period with the beads, all media were removed and the gels were washed three times with 500 μl of PBS. Trypsin-EDTA (200μl of 0.05%) was added to each well and the gels were incubated at 37°C for 15 minutes in 5% CO2. The cells were then collected into separate, sterile, 15ml conical tubes. Each tube was centrifuged for 8 minutes at 800 RPM, at room temperature. Excess media was removed from the conical tubes and the cells were resuspended in 3ml of PBS and 350μl of the resulting solution were transferred to flow cytometry tubes for processing. RPE suspensions were analyzed in a flow cytometer (BD FACSCanto II, BD Biosciences) to obtain the number of cells with phagocytosed beads. The signal intensity obtained from the beads allowed quantification of the number of cells that phagocytosed a fluorescent bead.
Three biological repeats were performed per substrate stiffness. Each gel was plated with a 30μl solution of 20,000 (cells/ml). ARPE-19 cells were studied at 14 days because the results from Guidry (2005) and Davis (Davis et al, 2012) suggested that that time scale was required for cells to adapt to a given substrate stiffness. We studied the cells after 4 hours of incubation with beads because, cultured RPE cells have been found to phagocytose rod outer segments at a steady rate for at least 3 hours, before decreasing their rate of phagocytosis (Kennedy et al, 1996).
Recent data (Provenzano et al, 2009) suggest that, for any given cell type, there are two cell culture regimes: ‘hard’ and ‘soft’. Therefore, cells cultured on glass substrates were characterized by markedly distorted cellular morphology and were not used, because their results would be less physiologically relevant.
Microscopy
Cells were imaged by epifluorescence and Hoffman illumination using a 20x objective on an inverted microscope (IX71; Olympus, Tokyo) and captured using a monochrome, cooled CCD digital camera (Rolera-XR; Q-Imaging, Surrey, BC, Canada). Epifluorescence images were captured at the optimal focal plane for the majority of beads (adherent cells) or at the surface of the cells (trypsinized, non-adherent cells) and multiple fields were sequentially captured. Digital images for the fluorescence (beads) and Hoffman illumination (cells) were over-laid to identify the beads associated with individual cells. Beads that were located on the cell surface or not associated with a cell demonstrated the brightest fluorescence and crisp boundaries. Beads that were inside a cell were not in the same focal plane as the cell surface and demonstrated dimmer fluorescence with blurred outlines.
Using these criteria we found that prior to trypsinization 2.1294% (p=0.0012) cells contained an internalized bead. Results were analyzed using an unpaired Student’s t-test and are representative of 3 independent experiments. This is consistent with the results from flow cytometry where 5.83% (583 out of 10,000) cells cultured on substrates of the same elastic modulus internalized a bead.
Data Analysis
The number of cells that phagocytosed a bead was corrected for the total number of events analyzed. Only cells with one phagocytosed bead were analyzed.
Out of 10,000 events counted, approximately 70% of the events had a form factor consistent with a single cell and only approximately 6% of all the events were consistent with a cell with one or more beads. Of those, consistently, less than 10% of the cells with one or more beads had more than one bead. This means that less than 0.6% of events were consistent with a cell containing more than one bead. This is on the order of magnitude of our measurement error and thus analysis of cells that have phagocytosed only one bead is appropriate. In addition, a cell containing more than one strongly-scattering bead will have an abnormal form factor and excluding counts with an abnormal form factor in our analysis also allows us to exclude cellular debris that is adherent to one or more beads.
Linear regression was performed to evaluate the dependence of RPE cell phagocytosis on varying substrate stiffness. The forward scatter channel (FSC) and side scatter channel (SSC) were gated to select viable cells of interest (Rahman, 2006). ARPE-19 cells not exposed to latex beads served as the negative control, and facilitated determination of the negative threshold in the fluorescence channel. Events exceeding the threshold and consistent with the fluorescence of a single bead were counted as phagocytosing cells.
Results
ARPE-19 cells demonstrated decreased phagocytosis on higher elastic modulus gels and a linear relationship between substrate elastic modulus and phagocytosis (Figure 1). ARPE-19 cells cultured on the highest elastic modulus exhibited the least amount of phagocytosis.
Figure 1.
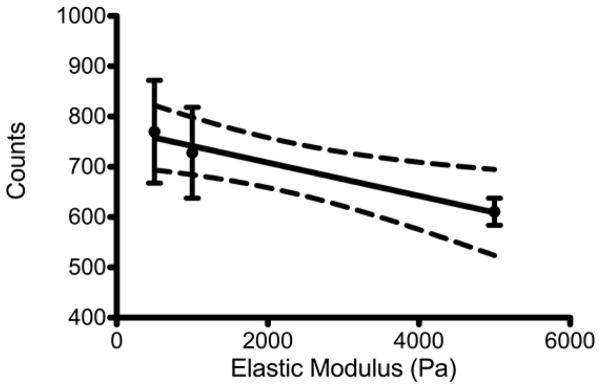
The number of ARPE-19 cells that phagocytosed a bead, per 10,000 cells (Counts) plotted versus the substrate stiffness (Elastic Modulus). The mean and standard error of the mean plotted with the results of a linear regression analysis. The dashed lines indicate 95% confidence intervals; p=0.0135; R2=0.4726.
Fluorescence microscopy images indicate that the latex beads are phagocytosed. Given that we only analyzed cells that are associated with one bead (ie, are characterized by the form factor of a cell and the fluorescence of a single bead), we avoided problems associated with multiple beads being phagocytosed or beads adherent to the cell surface.
Discussion
The results of this study, together with prior results (Janmey et al, 2009; Pelham and Wang, 1998; Pelham and Wang, 1998; Davis et al, 2012; Raghunathan et al, 2013; Georges et al, 2007), suggest that substrate elastic modulus influences cell behavior, including complex cell behaviors such as phagocytosis, in a variety of cell types.
Currently, there is substantial interest in developing synthetic substitutes for Bruch’s membrane for the culture of stem-cell derived RPE cells, for eventual transplantation into patients suffering from macular degeneration (Bharti et al, 2010). Our study suggests that the elastic modulus of these substrates may be a critical design parameter. In addition, sub-retinal implantation of prosthetic devices has recently come into clinical use and, in designing the device-tissue interface, the stiffness of the device surface should be considered.
A weakness of this study is the use of a cell line, rather than primary RPE cells. However, it is known that a variety of cell types including retinal Müller cells (Davis, 2012), primary human trabecular meshwork cells (Raghunathan et al, 2013), kidney epithelial cells (Pelham and Wang, 1998), and liver cells (Georges et al, 2007) and numerous cellular processes, including cell spreading and motility (Georges et al, 2005) are influenced by substrate elastic modulus.
Decreased phagocytosis by ARPE-19 cells cultured on substrates of higher elastic moduli suggests that changes in the stiffness of Bruch’s membrane may affect RPE cells in vivo. As Bruch’s membrane ages, RPE cells may be less able to phagocytose the outer segments of photoreceptor cells, and this may eventually lead to RPE dysfunction. In turn, RPE dysfunction may affect photoreceptor function, causing the loss of central vision in the macula.
Figure 2.
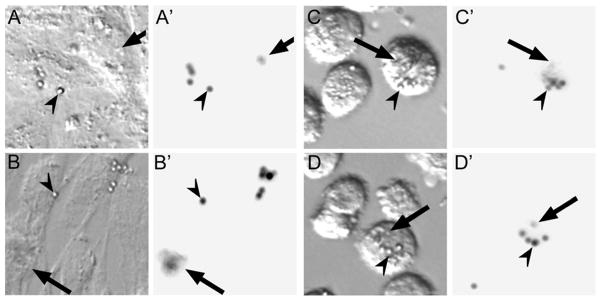
Imaging of ARPE-19 cells, grown on a 1000 Pa gel, after incubation with beads. A&B: phase contrast image before trypsinization, A′&B′: fluorescence image before trypsinization, C&D: phase contrast images after trypsinization, C′&D′: fluorescence images after trypsinization. Arrowheads indicate beads that were not phagocytosed while arrows indicate beads that have been phagocytosed by the RPE cells.
Acknowledgments
WJF acknowledges the support from the NEI/NIH (K08EY017112 and P30EY007551) and a National Academies Keck Futures Initiative Grant. AMM acknowledges support from the NEI/NIH (R01EY013175 and P30EY007551). The study sponsors had no role in the study design, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interests regarding the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bharti K, Miller SS, Arnheiter H. The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Research. 2010;24:21–34. doi: 10.1111/j.1755-148X.2010.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Wassell J. Chapter 4: Ageing of the human retinal pigment epithelium. In: Coscas G, Piccolino FC, editors. Retinal Pigment Epithelium and Macular Diseases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 19–28. [Google Scholar]
- Davis JT, Wen Q, Janmey PA, Otteson DC, Foster WJ. Müller cell expression of genes implicated in proliferative vitreoretinopathy is influenced by substrate elastic modulus. Investigative Ophthalmology and Visual Science. 2012;53:3014–3019. doi: 10.1167/iovs.11-8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KC. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Experimental Eye Research. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Fischer RF. The Influence of Age on Some Ocular Basement Membranes. Eye. 1987;1:184–189. doi: 10.1038/eye.1987.35. [DOI] [PubMed] [Google Scholar]
- Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. American Journal of Physiology Gastrointestinal and Liver Physiology. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. Journal of Applied Physiology. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- Green WR. Histopathology of features age-related macular degeneration. Molecular Vision. 1999;5:27. [PubMed] [Google Scholar]
- Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24:75–86. doi: 10.1016/j.preteyeres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Winer JP, Murray ME, Wen Q. The hard life of soft cells. Cell Motility and the Cytoskeleton. 2009;66:597–605. doi: 10.1002/cm.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CJ, Rakoczy PE, Constable IJ. A simple flow cytometric technique to quantify rod outer segment phagocytosis in cultured retinal pigment epithelial cells. Current Eye Research. 1996;15:998–1003. doi: 10.3109/02713689609017646. [DOI] [PubMed] [Google Scholar]
- Kevany B, Palczewski K. Phagocytosis of Retinal Rod and Cone Photoreceptors. Physiology. 2010;25:8–10. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Preparation of a Flexible, Porous Polyacrylamide Substrate for Mechanical Studies of Cultured Cells. Methods in Enzymology. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Cell Locomotion and Focal Adhesions Are Regulated by Substrate Flexibility. Proceedings of the National Academy of Sciences. 1998;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PH. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Dreier B, Reilly CM, Thomasy SM, Wood JA, Ly I, Tuyen BC, Hughbanks M, Murphy CJ, Russell P. Role of Substratum Stiffness in Modulating Genes Associated with Extracellular Matrix and Mechanotransducers YAP and TAZ. Investigative Ophthalmology and Visual Science. 2013;54:378–386. doi: 10.1167/iovs.12-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. Introduction to Flow Cytometry. Oxford: Serotec Ltd; 2006. pp. 4–8. [Google Scholar]


