Table 1.
Affinity of Pyridazinoindoles
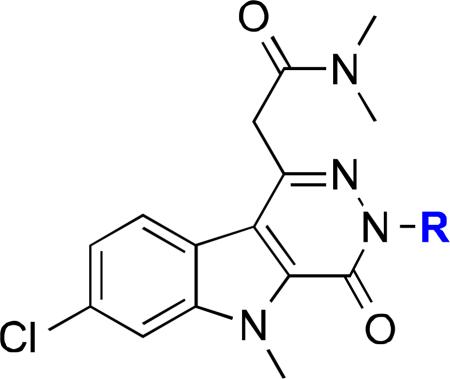
| ||||
|---|---|---|---|---|
| Compd | R | C6 Glioma Ki (pM)b | Heart Ki (pM)b | Kidney Ki (pM)b |
| 3a | Ph | 1.23 ± 0.08 | 0.762 ± 0.15 | 0.596 ± 0.04 |
| 9 | 2-fluorophenyl | 0.422 ± 0.06 | 0.591 ± 0.10 | 1.18 ± 0.04 |
| 10 | 3-fluorophenyl | 0.280 ± 0.07 | 0.180 ± 0.02 | 0.212 ± 0.02 |
| 11 | 3-nitrophenyl | 0.889 ± 0.2 | 1.18 ± 0.09 | 0.678 ± 0.2 |
| 12 | 4-fluorophenyl | 0.671 ± 0.2; 3020 ± 1007d | 0.877 ± 0.03 | 0.459 ± 0.03; 969 ± 752d |
| 13 | 2-pyridyl | 2409 ± 795.4 | 4936 ± 662.3 | 6796 ± 830.9 |
| 14c | 3-fluoro-2-pyridyl | |1.19 ± 0.05; 1770 ± 232.6d | 3.21 ± 0.4 | 2.21 ± 0.4 |
| 15 | 3-chloro-2-pyridyl | 2.34 ± 0.4 | 0.676 ± 0.08 | 0.495 ± 0.03 |
| 16 | 3-bromo-2-pyridyl | 1.48 ± 0.2 | 0.281 ± 0.01 | 0.965 ± 0.02 |
SSR180575.
Ki ± S.E.M. versus [3H]PK11195.
Ki versus [3H]flunitrazepam in rat brain lysate >10 μM.
Mixed affinity binding. All lysates procured from athymic nude rats. All experiments performed in triplicate.
