Abstract
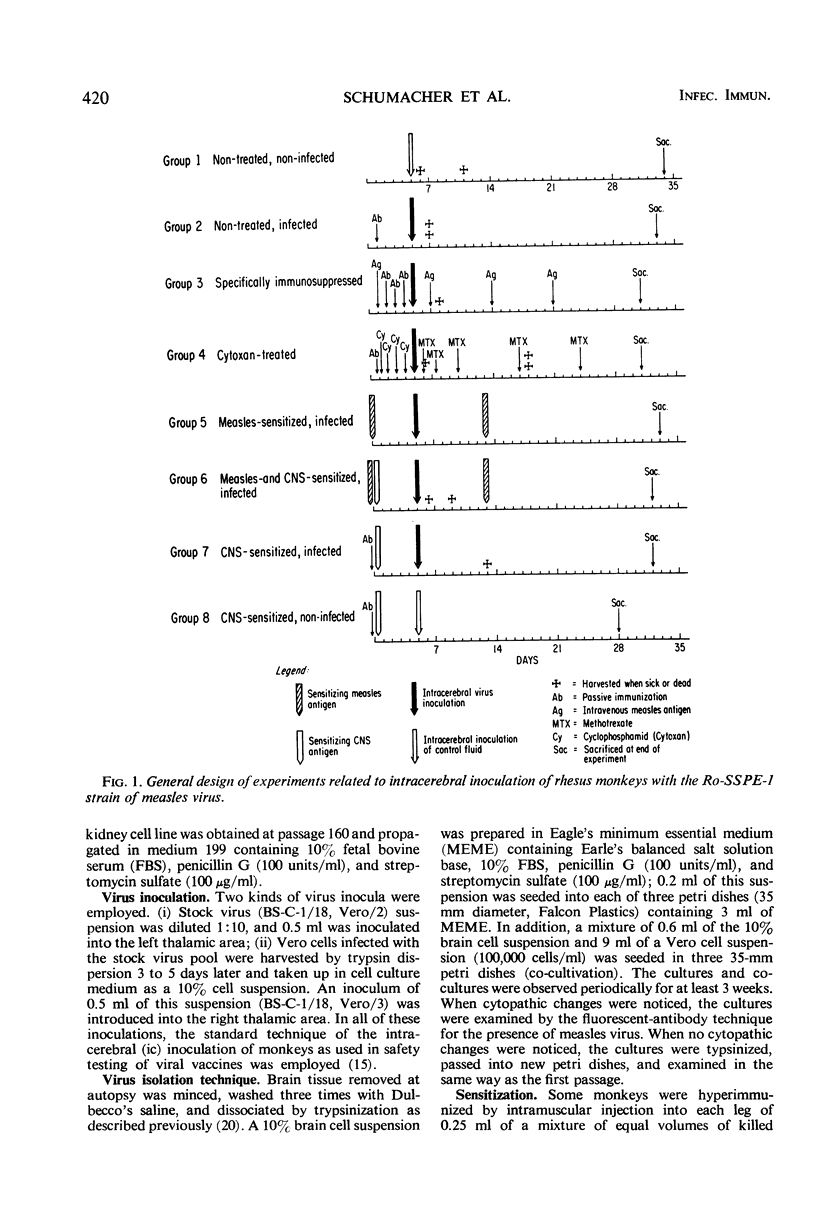
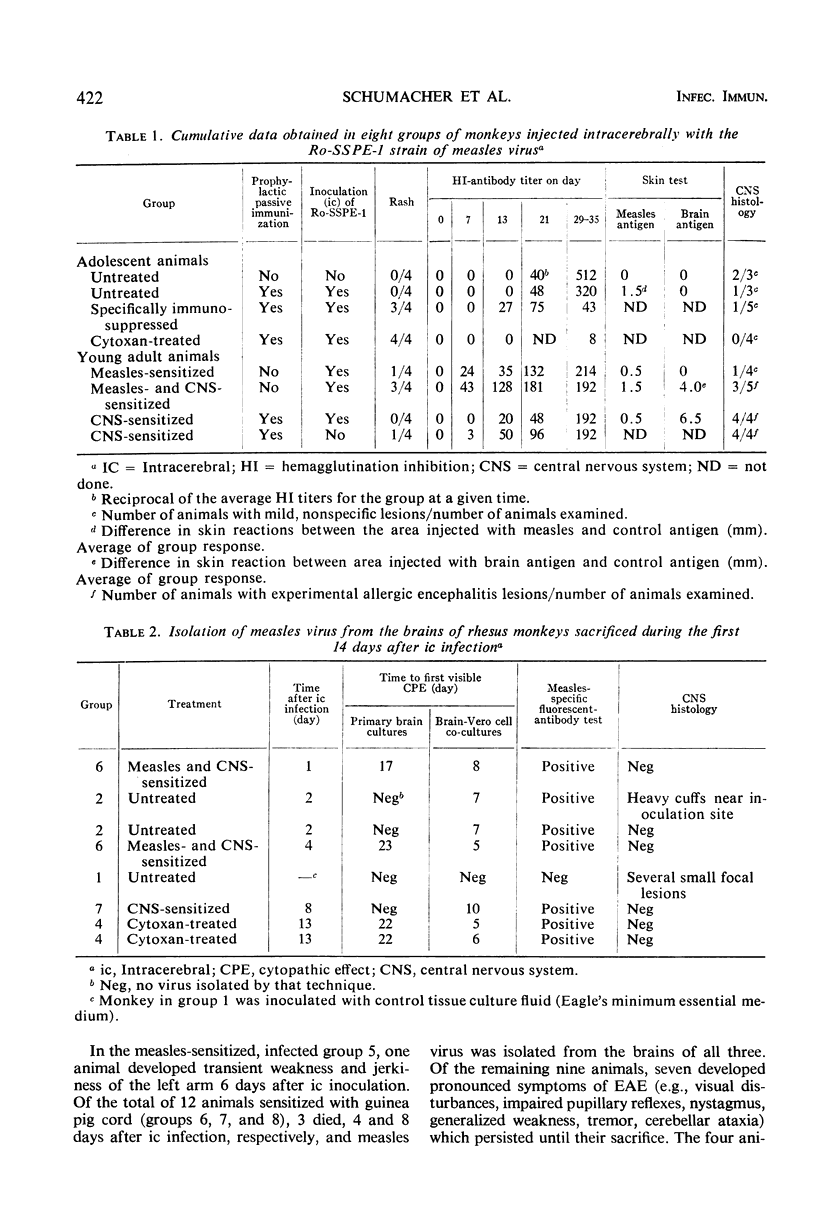
Measles virus isolated from the brain of a patient with subacute sclerosing panencephalitis was injected intracerebrally (ic) into 34 rhesus monkeys. Groups of these animals were injected with measles antigen in Freund's complete adjuvant or treated by schedules used for suppression of the general or cell-mediated immune responsiveness. In another group of animals, experimental allergic encephalitis was induced parallel with measles infection. Measles virus was isolated from the brains of monkeys up to 13 days after ic inoculation. No virus was detected in the central nervous system after 3 to 4 weeks, the longest postinoculation period examined. It was concluded that the subacute sclerosing panencephalitis-derived virus either lost its neurotropic properties at the passage level at which it was used or that it submerged into a silent stage and escaped detection. Neither immunosuppression nor concomitant autoimmune encephalitis had an effect on the survival of measles virus in the central nervous system. The histology of the nervous tissue was basically normal except for characteristic lesions of experimental allergic encephalitis in animals receiving the respective treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Baird C., Filloy L. Inclusion bodies in measles encephalitis. JAMA. 1966 Jan 24;195(4):290–298. [PubMed] [Google Scholar]
- Appel M. J. Pathogenesis of canine distemper. Am J Vet Res. 1969 Jul;30(7):1167–1182. [PubMed] [Google Scholar]
- Asherson G. L., Stone S. H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. I. Immune deviation: description of the phenomenon and the effect of splenectomy. Immunology. 1965 Sep;9(3):205–217. [PMC free article] [PubMed] [Google Scholar]
- Axelrad M. A. Suppression of delayed hypersensitivity by antigen and antibody. Is a common precursor cell responsible for both delayed hypersensitivity and antibody formation? Immunology. 1968 Aug;15(2):159–171. [PMC free article] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Oyanagi S., Katz M., Koprowski H. Presence of 2 different viral agents in brain cells of patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1970 May;134(1):230–236. doi: 10.3181/00379727-134-34765. [DOI] [PubMed] [Google Scholar]
- Baublis J. V., Payne F. E. Measles antigen and syncytium formtion in brain cell cultures from subacute sclerosing panencephalitis (SSPE). Proc Soc Exp Biol Med. 1968 Nov;129(2):593–597. doi: 10.3181/00379727-129-33377. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Hamilton R., Traub R., Ley A., Sever J. L. Some characteristics of SSPE measles virus. Proc Soc Exp Biol Med. 1970 May;134(1):17–21. doi: 10.3181/00379727-134-34718. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., London W. T., Jabbour J. T., Zeman W., Sever J. L. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969 Oct;132(1):272–277. doi: 10.3181/00379727-132-34196. [DOI] [PubMed] [Google Scholar]
- KOPROWSKI H. The role of hyperergy in measles encephalitis. Am J Dis Child. 1962 Mar;103:273–278. doi: 10.1001/archpedi.1962.02080020285019. [DOI] [PubMed] [Google Scholar]
- Katz M., Rorke L. B., Masland W. S., Brodano G. B., Koprowski H. Subacute sclerosing panencephalitis: isolation of a virus encephalitogenic for ferrets. J Infect Dis. 1970 Feb;121(2):188–195. doi: 10.1093/infdis/121.2.188. [DOI] [PubMed] [Google Scholar]
- MITUS A., ENDERS J. F., CRAIG J. M., HOLLOWAY A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med. 1959 Oct 29;261:882–889. doi: 10.1056/NEJM195910292611802. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- Paterson P. Y. Immune processes and infectious factors in central nervous system disease. Annu Rev Med. 1969;20:75–100. doi: 10.1146/annurev.me.20.020169.000451. [DOI] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Schumacher H. P., Albrecht P. Optimal conditions for isolation of a neurotropic measles virus from brain tissue. Proc Soc Exp Biol Med. 1970 Jun;134(2):396–402. doi: 10.3181/00379727-134-34799. [DOI] [PubMed] [Google Scholar]
- Scott T. F. Postinfectious and vaccinal encephalitis. Med Clin North Am. 1967 May;51(3):701–717. doi: 10.1016/s0025-7125(16)33039-5. [DOI] [PubMed] [Google Scholar]


