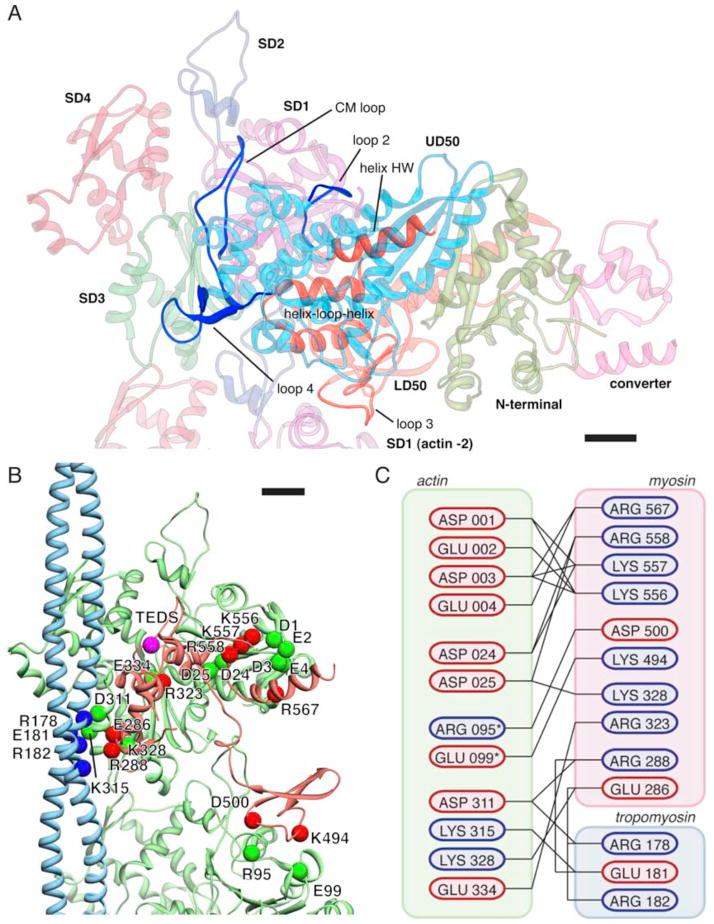
Figure 4. The binding interface with potential key electrostatic interactions between myosin, tropomyosin and actin(0) and (−2).
(A) Overview of the binding interface between myosin and actin. Regions on myosin involved in actin binding are highlighted and labeled. (B) Pseudo-atomic model of the complete binding interface. Potential interaction partners with complimentary charges in close proximity are depicted as colored spheres. In addition, the TEDS site (which is not part of the interface) is depicted as a pink sphere. The interface between actin(0) and myosin extends over 1,450 Å2, the interface between actin(−2) and myosin over 370 Å2, the interface between actin(0) and tropomyosin over 210 Å2 and the interface between myosin and tropomyosin over 300 Å2. (C) Cartoon representation of the interface. Residues are colored by charge at pH 7.4. Asterisk denotes residues that are part of the actin (−2) interface. Scale bars, 1 nm.