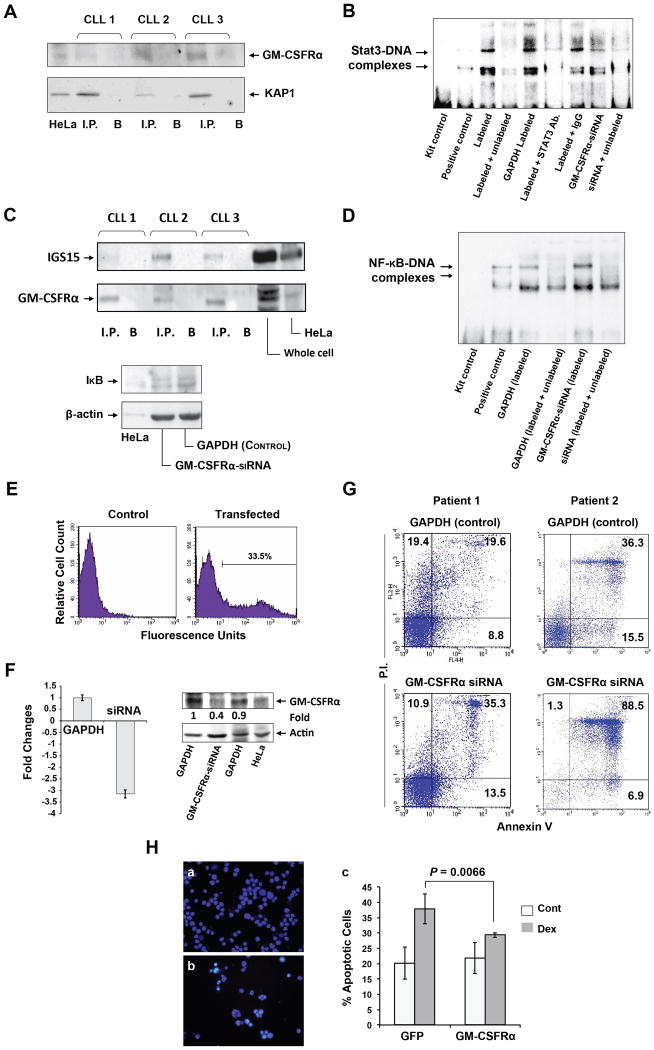
Figure 7.
GM-CSFRα protects cells from apoptotic cell death. (A) KAP1 co-immunoprecipitates with anti-GM-CSFRα antibodies. Chronic lymphocytic leukemia (CLL) nuclear extracts from 3 CLL patients were immunoprecipitated (I.P.) with anti-GM-CSFRα antibodies using protein A–agarose beads. Incubation with beads only (B) was used as a negative control. The immune complex was separated by SDS-PAGE and analyzed by Western immunoblotting using the indicated antibodies. (B) GM-CSFRα-siRNA reduced the binding of CLL nuclear protein to the labeled STAT3 DNA binding-site. EMSA was conducted using a biotin-labeled DNA probe harboring a STAT3 binding-site. The binding was significantly attenuated by excess unbiotinylated (cold) probes or anti-STAT3 antibodies. The binding of nuclear extract from CLL cells transfected with GM-CSFRα-siRNA was significantly reduced as compared with its transfection control cells (GAPDH), and the binding was reversed by excess cold probes. (C) Upper panel: GM-CSFRα co-immunoprecipitates with ISG15. Nuclear extracts from cells of 3 CLL patients were immunoprecipitated (I.P.) with anti-ISG15 antibodies using protein A–agarose beads. Incubation with beads only (B) was used as a negative control. Lower panel: GM-CSFRα-siRNA downregulates IκB protein levels. Low-density peripheral blood (PB) CLL cells were transfected with GM-CSFRα-siRNA or GAPDH, and IκB protein levels were assessed by Western immunoblotting. (D) Transfection of CLL cells with GM-CSFRα-siRNA enhances NF-κB-DNA binding. EMSA results of CLL nuclear protein and a labeled NF-κB DNA-binding probe demonstrate a greater binding of protein from CLL cells transfected with GM-CSFRα-siRNA than from CLL cells transfected with GAPDH. Excess cold probes partially reversed the binding, proving its specificity. (E–G) GM-CSFRα-siRNA induced apoptosis in CLL cells. (E) CLL cells were co-transfected with GFP and GAPDH or GM-CSFRα-siRNA at a transfection efficiency of 33.5%, as assessed by flow cytometry. (F) Transfection of CLL cells with GM-CSFRα-siRNA but not with GAPDH downregulates GM-CSFRα mRNA levels as assessed by qRT-PCR of 4 different experiments (left panel) and GM-CSFRα protein levels (right panel). (G) A higher apoptosis rate was observed in CLL cells transfected with GM-CSFRα-siRNA than in cells transfected with GAPDH after incubation for 24 hours in 10% FCS. Data obtained from 2 different patients are depicted. (H) GM-CSFRα-siRNA protects multiple myeloma cell line MM1 cells from dexamethasone-induced apoptosis. MM1 cells were transfected with GFP or GFP-tagged GM-CSFRα at a transfection efficiency of 40% (a, b). The transfected cells were incubated for 48 hours with or without 20 μmol/l of dexamethasone. Apoptosis rates were assessed by flow cytometry using Annexin V and PI. This experiment was repeated 3 times. Rates of apoptosis are depicted as mean ± SD. To assess the statistical difference between the variables, we used Student's t-test.