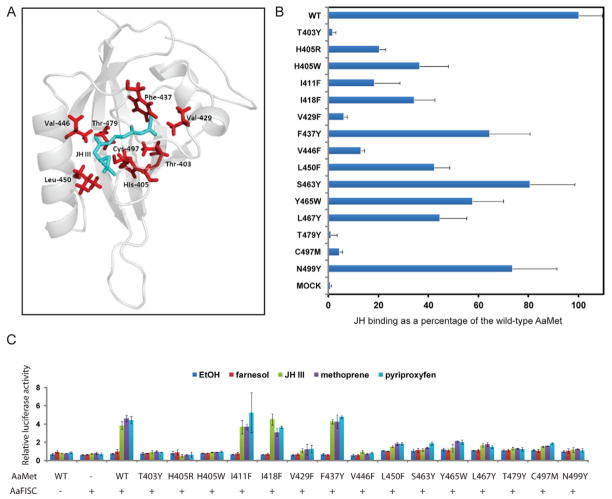
Figure 6.
Generation of JH-binding deficient mutants of AaMet by structure-guided mutagenesis. (A) Cartoon representation of the modeled AaMet PAS-B domain with the lowest-energy docked conformation of JH-III (shown as green sticks). (B) Effect of the mutations within the PAS-B domain of AaMet on the binding of [3H]-labeled JH-III. The binding was normalized against the AaMet inputs measured in Figure S3. (C) The JH-induced transactivation activity decreased substantially in the JH-binding deficient mutants of AaMet. Drosophila L57 cells were transfected by the 4×JHRE1-luc reporter plasmid and the expression vectors for AaFISC and the indicated AaMet derivatives. The mutations in AaMet did not markedly affect the expression of AaMet and AaFISC in L57 cells (Fig. S4). Transfected cells were treated with 1 μM of farnesol, JH-III, methoprene or pyriproxyfen. Methoprene and pyriproxyfen are two JH agonists. Farnesol is a biosynthetic intermediate for JH-III. Ethanol was used as control. The mean and standard deviation from triplicate samples were indicated.